[English] 日本語
 Yorodumi
Yorodumi- EMDB-23222: Designed tetrahedral nanoparticle T33-31 presenting BG505 SOSIP t... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23222 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
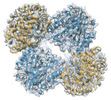
| Title | Designed tetrahedral nanoparticle T33-31 presenting BG505 SOSIP trimers | |||||||||
 Map data Map data | BG505 SOSIP-T33-31 nanoparticle - Main map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | HIV / vaccine design / protein design / nanoparticles / BG505 / VIRAL PROTEIN / DE NOVO PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / virus-mediated perturbation of host defense response / fusion of virus membrane with host endosome membrane / viral envelope ...positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / virus-mediated perturbation of host defense response / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / apoptotic process / host cell plasma membrane / structural molecule activity / virion membrane / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |   Human immunodeficiency virus 1 Human immunodeficiency virus 1 | |||||||||
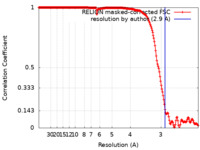
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Antanasijevic A / Sewall LM | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Polyclonal antibody responses to HIV Env immunogens resolved using cryoEM. Authors: Aleksandar Antanasijevic / Leigh M Sewall / Christopher A Cottrell / Diane G Carnathan / Luis E Jimenez / Julia T Ngo / Jennifer B Silverman / Bettina Groschel / Erik Georgeson / Jinal ...Authors: Aleksandar Antanasijevic / Leigh M Sewall / Christopher A Cottrell / Diane G Carnathan / Luis E Jimenez / Julia T Ngo / Jennifer B Silverman / Bettina Groschel / Erik Georgeson / Jinal Bhiman / Raiza Bastidas / Celia LaBranche / Joel D Allen / Jeffrey Copps / Hailee R Perrett / Kimmo Rantalainen / Fabien Cannac / Yuhe R Yang / Alba Torrents de la Peña / Rebeca Froes Rocha / Zachary T Berndsen / David Baker / Neil P King / Rogier W Sanders / John P Moore / Shane Crotty / Max Crispin / David C Montefiori / Dennis R Burton / William R Schief / Guido Silvestri / Andrew B Ward /    Abstract: Engineered ectodomain trimer immunogens based on BG505 envelope glycoprotein are widely utilized as components of HIV vaccine development platforms. In this study, we used rhesus macaques to evaluate ...Engineered ectodomain trimer immunogens based on BG505 envelope glycoprotein are widely utilized as components of HIV vaccine development platforms. In this study, we used rhesus macaques to evaluate the immunogenicity of several stabilized BG505 SOSIP constructs both as free trimers and presented on a nanoparticle. We applied a cryoEM-based method for high-resolution mapping of polyclonal antibody responses elicited in immunized animals (cryoEMPEM). Mutational analysis coupled with neutralization assays were used to probe the neutralization potential at each epitope. We demonstrate that cryoEMPEM data can be used for rapid, high-resolution analysis of polyclonal antibody responses without the need for monoclonal antibody isolation. This approach allowed to resolve structurally distinct classes of antibodies that bind overlapping sites. In addition to comprehensive mapping of commonly targeted neutralizing and non-neutralizing epitopes in BG505 SOSIP immunogens, our analysis revealed that epitopes comprising engineered stabilizing mutations and of partially occupied glycosylation sites can be immunogenic. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23222.map.gz emd_23222.map.gz | 502.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23222-v30.xml emd-23222-v30.xml emd-23222.xml emd-23222.xml | 21.4 KB 21.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_23222_fsc.xml emd_23222_fsc.xml | 18.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_23222.png emd_23222.png | 279.8 KB | ||
| Masks |  emd_23222_msk_1.map emd_23222_msk_1.map | 536.4 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-23222.cif.gz emd-23222.cif.gz | 6.7 KB | ||
| Others |  emd_23222_half_map_1.map.gz emd_23222_half_map_1.map.gz emd_23222_half_map_2.map.gz emd_23222_half_map_2.map.gz | 432.2 MB 432.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23222 http://ftp.pdbj.org/pub/emdb/structures/EMD-23222 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23222 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23222 | HTTPS FTP |
-Validation report
| Summary document |  emd_23222_validation.pdf.gz emd_23222_validation.pdf.gz | 743.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_23222_full_validation.pdf.gz emd_23222_full_validation.pdf.gz | 743.4 KB | Display | |
| Data in XML |  emd_23222_validation.xml.gz emd_23222_validation.xml.gz | 25.4 KB | Display | |
| Data in CIF |  emd_23222_validation.cif.gz emd_23222_validation.cif.gz | 33.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23222 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23222 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23222 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23222 | HTTPS FTP |
-Related structure data
| Related structure data |  7l85MC  7l7tC  7l7uC  7l86C  7l87C  7l88C  7l89C  7l8aC  7l8bC  7l8cC  7l8dC  7l8eC  7l8fC  7l8gC  7l8sC  7l8tC  7l8uC  7l8wC  7l8xC  7l8yC  7l8zC  7l90C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23222.map.gz / Format: CCP4 / Size: 536.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23222.map.gz / Format: CCP4 / Size: 536.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BG505 SOSIP-T33-31 nanoparticle - Main map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.15 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_23222_msk_1.map emd_23222_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: BG505 SOSIP-T33-31 nanoparticle - Half-map 1
| File | emd_23222_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BG505 SOSIP-T33-31 nanoparticle - Half-map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: BG505 SOSIP-T33-31 nanoparticle - Half-map 2
| File | emd_23222_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BG505 SOSIP-T33-31 nanoparticle - Half-map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Designed tetrahedral nanoparticle BG505 SOSIP-T33-31
| Entire | Name: Designed tetrahedral nanoparticle BG505 SOSIP-T33-31 |
|---|---|
| Components |
|
-Supramolecule #1: Designed tetrahedral nanoparticle BG505 SOSIP-T33-31
| Supramolecule | Name: Designed tetrahedral nanoparticle BG505 SOSIP-T33-31 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: The map was generated by focused refinement of BG505 SOSIP-T33-31 nanoparticle dataset using a mask around the T33-31 nanoparticle core (masking out the flexibly linked antigens). |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
-Macromolecule #1: BG505 SOSIP-T33-31B
| Macromolecule | Name: BG505 SOSIP-T33-31B / type: protein_or_peptide / ID: 1 / Number of copies: 12 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Molecular weight | Theoretical: 13.679698 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GSVRGIRGAI TVEEDTPAAI LAATIELLLK MLEANGIQSY EELAAVIFTV TEDLTSAFPA EAARLIGMHR VPLLSAREVP VPGSLPRVI RVLALWNTDT PQDRVRHVYL NEAVRLRPDL ESAQLE |
-Macromolecule #2: BG505 SOSIP-T33-31A
| Macromolecule | Name: BG505 SOSIP-T33-31A / type: protein_or_peptide / ID: 2 / Number of copies: 12 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Molecular weight | Theoretical: 11.698429 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GGEEVVLITV PSALVAVKIA HALVEERLAA CVNIVPGLTS IYREEGSVVS DHELLLLVKT TTDAFPKLKE RVKELHPYEV PEIVALPIA EGNREYLDWL RENTGLE |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4.1 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: TBS buffer prepared from a 10X stock | |||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 10 sec. / Pretreatment - Atmosphere: OTHER | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 283 K / Instrument: FEI VITROBOT MARK IV / Details: Blotting time varied between 3 and 7 seconds.. | |||||||||
| Details | BG505 SOSIP-T33-31 nanoparticle was prepared by combining equimolar amounts of BG505 SOSIP-T33-31A and BG505 SOSIP-T33-31B components that were expressed separately. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-41 / Number grids imaged: 2 / Number real images: 1751 / Average exposure time: 10.25 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 36000 |
| Sample stage | Specimen holder model: OTHER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)