+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23131 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of full-length TRPV1 at pH6b state | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | TRP channel / cryo-EM / nanodisc / full length / proton / TRANSPORT PROTEIN | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of iodide transmembrane transport / positive regulation of membrane depolarization / negative regulation of establishment of blood-brain barrier / response to capsazepine / sensory perception of mechanical stimulus / cellular response to temperature stimulus / peptide secretion / smooth muscle contraction involved in micturition / positive regulation of sensory perception of pain / temperature-gated ion channel activity ...negative regulation of iodide transmembrane transport / positive regulation of membrane depolarization / negative regulation of establishment of blood-brain barrier / response to capsazepine / sensory perception of mechanical stimulus / cellular response to temperature stimulus / peptide secretion / smooth muscle contraction involved in micturition / positive regulation of sensory perception of pain / temperature-gated ion channel activity / detection of chemical stimulus involved in sensory perception of pain / positive regulation of renal sodium excretion / TRP channels / negative regulation of axon regeneration / positive regulation of cardiac muscle cell differentiation / fever generation / excitatory extracellular ligand-gated monoatomic ion channel activity / urinary bladder smooth muscle contraction / detection of temperature stimulus involved in thermoception / thermoception / negative regulation of systemic arterial blood pressure / response to pH / dendritic spine membrane / glutamate secretion / monoatomic cation transmembrane transporter activity / positive regulation of urine volume / response to acidic pH / cellular response to acidic pH / negative regulation of heart rate / response to pain / diet induced thermogenesis / cellular response to alkaloid / temperature homeostasis / cellular response to ATP / cellular response to cytokine stimulus / detection of temperature stimulus involved in sensory perception of pain / ligand-gated monoatomic ion channel activity / intracellularly gated calcium channel activity / behavioral response to pain / negative regulation of mitochondrial membrane potential / calcium ion import across plasma membrane / positive regulation of vasoconstriction / monoatomic ion channel activity / monoatomic cation channel activity / extracellular ligand-gated monoatomic ion channel activity / phosphatidylinositol binding / sensory perception of pain / axon terminus / positive regulation of excitatory postsynaptic potential / sarcoplasmic reticulum / lipid metabolic process / phosphoprotein binding / microglial cell activation / cellular response to nerve growth factor stimulus / response to peptide hormone / calcium ion transmembrane transport / cellular response to growth factor stimulus / GABA-ergic synapse / calcium channel activity / positive regulation of nitric oxide biosynthetic process / cellular response to tumor necrosis factor / calcium ion transport / transmembrane signaling receptor activity / sensory perception of taste / cellular response to heat / response to heat / positive regulation of cytosolic calcium ion concentration / monoatomic ion transmembrane transport / protein homotetramerization / postsynaptic membrane / calmodulin binding / neuron projection / positive regulation of apoptotic process / external side of plasma membrane / neuronal cell body / dendrite / negative regulation of transcription by RNA polymerase II / ATP binding / metal ion binding / identical protein binding / membrane / nucleus / plasma membrane Similarity search - Function | ||||||||||||||||||
| Biological species |  | ||||||||||||||||||
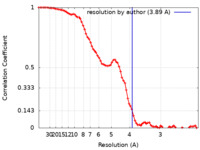
| Method | single particle reconstruction / cryo EM / Resolution: 3.89 Å | ||||||||||||||||||
 Authors Authors | Zhang K / Julius D | ||||||||||||||||||
| Funding support |  France, France,  United States, 5 items United States, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Cell / Year: 2021 Journal: Cell / Year: 2021Title: Structural snapshots of TRPV1 reveal mechanism of polymodal functionality. Authors: Kaihua Zhang / David Julius / Yifan Cheng /  Abstract: Many transient receptor potential (TRP) channels respond to diverse stimuli and conditionally conduct small and large cations. Such functional plasticity is presumably enabled by a uniquely dynamic ...Many transient receptor potential (TRP) channels respond to diverse stimuli and conditionally conduct small and large cations. Such functional plasticity is presumably enabled by a uniquely dynamic ion selectivity filter that is regulated by physiological agents. What is currently missing is a "photo series" of intermediate structural states that directly address this hypothesis and reveal specific mechanisms behind such dynamic channel regulation. Here, we exploit cryoelectron microscopy (cryo-EM) to visualize conformational transitions of the capsaicin receptor, TRPV1, as a model to understand how dynamic transitions of the selectivity filter in response to algogenic agents, including protons, vanilloid agonists, and peptide toxins, permit permeation by small and large organic cations. These structures also reveal mechanisms governing ligand binding substates, as well as allosteric coupling between key sites that are proximal to the selectivity filter and cytoplasmic gate. These insights suggest a general framework for understanding how TRP channels function as polymodal signal integrators. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23131.map.gz emd_23131.map.gz | 40.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23131-v30.xml emd-23131-v30.xml emd-23131.xml emd-23131.xml | 14.5 KB 14.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_23131_fsc.xml emd_23131_fsc.xml | 7.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_23131.png emd_23131.png | 253.8 KB | ||
| Filedesc metadata |  emd-23131.cif.gz emd-23131.cif.gz | 5.6 KB | ||
| Others |  emd_23131_half_map_1.map.gz emd_23131_half_map_1.map.gz emd_23131_half_map_2.map.gz emd_23131_half_map_2.map.gz | 29.8 MB 29.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23131 http://ftp.pdbj.org/pub/emdb/structures/EMD-23131 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23131 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23131 | HTTPS FTP |
-Related structure data
| Related structure data |  7l2kMC  7l2hC  7l2iC  7l2jC  7l2lC  7l2mC  7l2nC  7l2oC  7l2pC  7l2rC  7l2sC  7l2tC  7l2uC  7l2vC  7l2wC  7l2xC  7mz5C  7mz6C  7mz7C  7mz9C  7mzaC  7mzbC  7mzcC  7mzdC  7mzeC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23131.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23131.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.15 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: #2
| File | emd_23131_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_23131_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : full-length TRPV1 in nanodisc
| Entire | Name: full-length TRPV1 in nanodisc |
|---|---|
| Components |
|
-Supramolecule #1: full-length TRPV1 in nanodisc
| Supramolecule | Name: full-length TRPV1 in nanodisc / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Transient receptor potential cation channel subfamily V member 1
| Macromolecule | Name: Transient receptor potential cation channel subfamily V member 1 type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 95.328156 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GAMGSEQRAS LDSEESESPP QENSCLDPPD RDPNCKPPPV KPHIFTTRSR TRLFGKGDSE EASPLDCPYE EGGLASCPII TVSSVLTIQ RPGDGPASVR PSSQDSVSAG EKPPRLYDRR SIFDAVAQSN CQELESLLPF LQRSKKRLTD SEFKDPETGK T CLLKAMLN ...String: GAMGSEQRAS LDSEESESPP QENSCLDPPD RDPNCKPPPV KPHIFTTRSR TRLFGKGDSE EASPLDCPYE EGGLASCPII TVSSVLTIQ RPGDGPASVR PSSQDSVSAG EKPPRLYDRR SIFDAVAQSN CQELESLLPF LQRSKKRLTD SEFKDPETGK T CLLKAMLN LHNGQNDTIA LLLDVARKTD SLKQFVNASY TDSYYKGQTA LHIAIERRNM TLVTLLVENG ADVQAAANGD FF KKTKGRP GFYFGELPLS LAACTNQLAI VKFLLQNSWQ PADISARDSV GNTVLHALVE VADNTVDNTK FVTSMYNEIL ILG AKLHPT LKLEEITNRK GLTPLALAAS SGKIGVLAYI LQREIHEPEC RHLSRKFTEW AYGPVHSSLY DLSCIDTCEK NSVL EVIAY SSSETPNRHD MLLVEPLNRL LQDKWDRFVK RIFYFNFFVY CLYMIIFTAA AYYRPVEGLP PYKLKNTVGD YFRVT GEIL SVSGGVYFFF RGIQYFLQRR PSLKSLFVDS YSEILFFVQS LFMLVSVVLY FSQRKEYVAS MVFSLAMGWT NMLYYT RGF QQMGIYAVMI EKMILRDLCR FMFVYLVFLF GFSTAVVTLI EDGKNNSLPM ESTPHKCRGS ACKPGNSYNS LYSTCLE LF KFTIGMGDLE FTENYDFKAV FIILLLAYVI LTYILLLNML IALMGETVNK IAQESKNIWK LQRAITILDT EKSFLKCM R KAFRSGKLLQ VGFTPDGKDD YRWCFRVDEV NWTTWNTNVG IINEDPGNCE GVKRTLSFSL RSGRVSGRNW KNFALVPLL RDASTRDRHA TQQEEVQLKH YTGSLKPEDA EVFKDSMVPG EK UniProtKB: Transient receptor potential cation channel subfamily V member 1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 6 |
|---|---|
| Sugar embedding | Material: nanodisc |
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 76.8 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)