+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22462 | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | EPYC1(106-135) peptide-bound Rubisco | ||||||||||||||||||||||||||||||
 Map data Map data | EPYC1(106-135) peptide-bound Rubisco | ||||||||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||||||||
 Keywords Keywords | Rubisco / Pyrenoid / PLANT PROTEIN / Lyase | ||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationphotorespiration / ribulose-bisphosphate carboxylase / ribulose-bisphosphate carboxylase activity / reductive pentose-phosphate cycle / chloroplast stroma / chloroplast / monooxygenase activity / magnesium ion binding Similarity search - Function | ||||||||||||||||||||||||||||||
| Biological species |  | ||||||||||||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.06 Å | ||||||||||||||||||||||||||||||
 Authors Authors | Chou H / Matthies D / He S / Jonikas MC / Yu Z | ||||||||||||||||||||||||||||||
| Funding support |  United States, United States,  Singapore, Singapore,  United Kingdom, 9 items United Kingdom, 9 items
| ||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Plants / Year: 2020 Journal: Nat Plants / Year: 2020Title: The structural basis of Rubisco phase separation in the pyrenoid. Authors: Shan He / Hui-Ting Chou / Doreen Matthies / Tobias Wunder / Moritz T Meyer / Nicky Atkinson / Antonio Martinez-Sanchez / Philip D Jeffrey / Sarah A Port / Weronika Patena / Guanhua He / ...Authors: Shan He / Hui-Ting Chou / Doreen Matthies / Tobias Wunder / Moritz T Meyer / Nicky Atkinson / Antonio Martinez-Sanchez / Philip D Jeffrey / Sarah A Port / Weronika Patena / Guanhua He / Vivian K Chen / Frederick M Hughson / Alistair J McCormick / Oliver Mueller-Cajar / Benjamin D Engel / Zhiheng Yu / Martin C Jonikas /     Abstract: Approximately one-third of global CO fixation occurs in a phase-separated algal organelle called the pyrenoid. The existing data suggest that the pyrenoid forms by the phase separation of the CO- ...Approximately one-third of global CO fixation occurs in a phase-separated algal organelle called the pyrenoid. The existing data suggest that the pyrenoid forms by the phase separation of the CO-fixing enzyme Rubisco with a linker protein; however, the molecular interactions underlying this phase separation remain unknown. Here we present the structural basis of the interactions between Rubisco and its intrinsically disordered linker protein Essential Pyrenoid Component 1 (EPYC1) in the model alga Chlamydomonas reinhardtii. We find that EPYC1 consists of five evenly spaced Rubisco-binding regions that share sequence similarity. Single-particle cryo-electron microscopy of these regions in complex with Rubisco indicates that each Rubisco holoenzyme has eight binding sites for EPYC1, one on each Rubisco small subunit. Interface mutations disrupt binding, phase separation and pyrenoid formation. Cryo-electron tomography supports a model in which EPYC1 and Rubisco form a codependent multivalent network of specific low-affinity bonds, giving the matrix liquid-like properties. Our results advance the structural and functional understanding of the phase separation underlying the pyrenoid, an organelle that plays a fundamental role in the global carbon cycle. | ||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22462.map.gz emd_22462.map.gz | 76.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22462-v30.xml emd-22462-v30.xml emd-22462.xml emd-22462.xml | 18 KB 18 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_22462_fsc.xml emd_22462_fsc.xml | 11.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_22462.png emd_22462.png | 96.3 KB | ||
| Filedesc metadata |  emd-22462.cif.gz emd-22462.cif.gz | 6.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22462 http://ftp.pdbj.org/pub/emdb/structures/EMD-22462 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22462 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22462 | HTTPS FTP |
-Validation report
| Summary document |  emd_22462_validation.pdf.gz emd_22462_validation.pdf.gz | 509.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_22462_full_validation.pdf.gz emd_22462_full_validation.pdf.gz | 509.3 KB | Display | |
| Data in XML |  emd_22462_validation.xml.gz emd_22462_validation.xml.gz | 12.2 KB | Display | |
| Data in CIF |  emd_22462_validation.cif.gz emd_22462_validation.cif.gz | 16.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22462 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22462 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22462 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22462 | HTTPS FTP |
-Related structure data
| Related structure data |  7jsxMC  7jfoC  7jn4C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10501 (Title: CryoEM structure of EPYC1(106-135) peptide-bound Rubisco EMPIAR-10501 (Title: CryoEM structure of EPYC1(106-135) peptide-bound RubiscoData size: 5.8 TB Data #1: Unaligned multuframe micrographs of EPYC1(106-135) peptide-bound Rubisco [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22462.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22462.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EPYC1(106-135) peptide-bound Rubisco | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.844 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
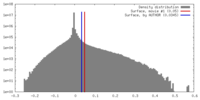
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : EPYC1(106-135) peptide-bound Rubisco
| Entire | Name: EPYC1(106-135) peptide-bound Rubisco |
|---|---|
| Components |
|
-Supramolecule #1: EPYC1(106-135) peptide-bound Rubisco
| Supramolecule | Name: EPYC1(106-135) peptide-bound Rubisco / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Ribulose bisphosphate carboxylase large chain
| Macromolecule | Name: Ribulose bisphosphate carboxylase large chain / type: protein_or_peptide / ID: 1 / Number of copies: 8 / Enantiomer: LEVO / EC number: ribulose-bisphosphate carboxylase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 52.607812 KDa |
| Sequence | String: MVPQTETKAG AGFKAGVKDY RLTYYTPDYV VRDTDILAAF RMTPQPGVPP EECGAAVAAE SSTGTWTTVW TDGLTSLDRY KGRCYDIEP VPGEDNQYIA YVAYPIDLFE EGSVTNMFTS IVGNVFGFKA LRALRLEDLR IPPAYVKTFV GPPHGIQVER D KLNKYGRG ...String: MVPQTETKAG AGFKAGVKDY RLTYYTPDYV VRDTDILAAF RMTPQPGVPP EECGAAVAAE SSTGTWTTVW TDGLTSLDRY KGRCYDIEP VPGEDNQYIA YVAYPIDLFE EGSVTNMFTS IVGNVFGFKA LRALRLEDLR IPPAYVKTFV GPPHGIQVER D KLNKYGRG LLGCTIKPKL GLSAKNYGRA VYECLRGGLD FTKDDENVNS QPFMRWRDRF LFVAEAIYKA QAETGEVKGH YL NATAGTC EEMMKRAV(SMC)A KELGVPIIMH DYLTGGFTAN TSLAIYCRDN GLLLHIHRAM HAVIDRQRNH GIHFRVLAK ALRMSGGDHL HSGTVVGKLE GEREVTLGFV DLMRDDYVEK DRSRGIYFTQ DWCSMPGVMP VASGGIHVWH MPALVEIFGD DACLQFGGG TLGHPWGNAP GAAANRVALE ACTQARNEGR DLAREGGDVI RSACKWSPEL AAACEVWKEI KFEFDTIDKL UniProtKB: Ribulose bisphosphate carboxylase large chain |
-Macromolecule #2: Ribulose bisphosphate carboxylase small chain 2, chloroplastic
| Macromolecule | Name: Ribulose bisphosphate carboxylase small chain 2, chloroplastic type: protein_or_peptide / ID: 2 / Number of copies: 8 / Enantiomer: LEVO / EC number: ribulose-bisphosphate carboxylase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 20.667959 KDa |
| Sequence | String: MAAVIAKSSV SAAVARPARS SVRPMAALKP AVKAAPVAAP AQANQMMVWT PVNNKMFETF SYLPPLSDEQ IAAQVDYIVA NGWIPCLEF AESDKAYVSN ESAIRFGSVS CLYYDNRYWT MWKLPMFGCR DPMQVLREIV ACTKAFPDAY VRLVAFDNQK Q VQIMGFLV QRPKSARDWQ PANKRSV UniProtKB: Ribulose bisphosphate carboxylase small subunit, chloroplastic 2 |
-Macromolecule #3: EPYC1
| Macromolecule | Name: EPYC1 / type: protein_or_peptide / ID: 3 / Number of copies: 8 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 3.294677 KDa |
| Sequence | String: RSSSASKKAV TPSRSALPSN WKQELESLRS UniProtKB: Uncharacterized protein |
-Macromolecule #4: water
| Macromolecule | Name: water / type: ligand / ID: 4 / Number of copies: 1141 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 6.8 Details: 200 mM sorbitol, 50 mM HEPES, 50 mM KOAc, 2 mM Mg(OAc)2.4H2O and 1 mM CaCl2 |
|---|---|
| Grid | Model: Quantifoil / Material: COPPER / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. Details: glow discharging for 60 seconds with a current of 15 mA in a Pelico EasiGlow system |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Dimensions - Width: 11520 pixel / Digitization - Dimensions - Height: 8184 pixel / Number real images: 13727 / Average exposure time: 3.56 sec. / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 0.0 mm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)