[English] 日本語
 Yorodumi
Yorodumi- EMDB-21453: SthK P300A cyclic nucleotide-gated potassium channel in the close... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21453 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | SthK P300A cyclic nucleotide-gated potassium channel in the closed state, in complex with cAMP | ||||||||||||
 Map data Map data | final map after 3D refinement | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | TRANSPORT PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationintracellularly cyclic nucleotide-activated monoatomic cation channel activity / protein-containing complex binding / membrane Similarity search - Function | ||||||||||||
| Biological species |  Spirochaeta thermophila (strain ATCC 700085 / DSM 6578 / Z-1203) (bacteria) Spirochaeta thermophila (strain ATCC 700085 / DSM 6578 / Z-1203) (bacteria) | ||||||||||||
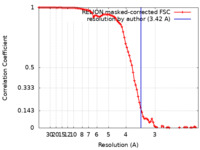
| Method | single particle reconstruction / cryo EM / Resolution: 3.42 Å | ||||||||||||
 Authors Authors | Schmidpeter PAM / Rheinberger J / Nimigean CM | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Prolyl isomerization controls activation kinetics of a cyclic nucleotide-gated ion channel. Authors: Philipp A M Schmidpeter / Jan Rheinberger / Crina M Nimigean /   Abstract: SthK, a cyclic nucleotide-modulated ion channel from Spirochaeta thermophila, activates slowly upon cAMP increase. This is reminiscent of the slow, cAMP-induced activation reported for the ...SthK, a cyclic nucleotide-modulated ion channel from Spirochaeta thermophila, activates slowly upon cAMP increase. This is reminiscent of the slow, cAMP-induced activation reported for the hyperpolarization-activated and cyclic nucleotide-gated channel HCN2 in the family of so-called pacemaker channels. Here, we investigate slow cAMP-induced activation in purified SthK channels using stopped-flow assays, mutagenesis, enzymatic catalysis and inhibition assays revealing that the cis/trans conformation of a conserved proline in the cyclic nucleotide-binding domain determines the activation kinetics of SthK. We propose that SthK exists in two forms: trans Pro300 SthK with high ligand binding affinity and fast activation, and cis Pro300 SthK with low affinity and slow activation. Following channel activation, the cis/trans equilibrium, catalyzed by prolyl isomerases, is shifted towards trans, while steady-state channel activity is unaffected. Our results reveal prolyl isomerization as a regulatory mechanism for SthK, and potentially eukaryotic HCN channels. This mechanism could contribute to electrical rhythmicity in cells. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21453.map.gz emd_21453.map.gz | 59.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21453-v30.xml emd-21453-v30.xml emd-21453.xml emd-21453.xml | 22.3 KB 22.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_21453_fsc.xml emd_21453_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_21453.png emd_21453.png | 115.1 KB | ||
| Masks |  emd_21453_msk_1.map emd_21453_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-21453.cif.gz emd-21453.cif.gz | 6.6 KB | ||
| Others |  emd_21453_additional_1.map.gz emd_21453_additional_1.map.gz emd_21453_half_map_1.map.gz emd_21453_half_map_1.map.gz emd_21453_half_map_2.map.gz emd_21453_half_map_2.map.gz | 45.4 MB 45.7 MB 45.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21453 http://ftp.pdbj.org/pub/emdb/structures/EMD-21453 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21453 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21453 | HTTPS FTP |
-Related structure data
| Related structure data |  6vxzMC  6vy0C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_21453.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21453.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | final map after 3D refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0961 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
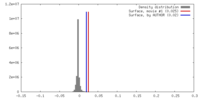
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_21453_msk_1.map emd_21453_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
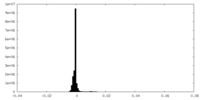
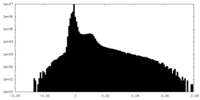
| Density Histograms |
-Additional map: final map after 3D refinement
| File | emd_21453_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | final map after 3D refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
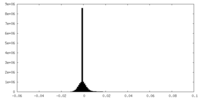
| Density Histograms |
-Half map: half map 1
| File | emd_21453_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
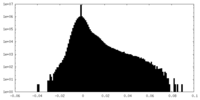
| Density Histograms |
-Half map: final map after 3D refinement
| File | emd_21453_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | final map after 3D refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
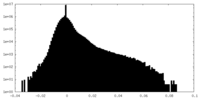
| Density Histograms |
- Sample components
Sample components
-Entire : SthK P300A bound to cAMP
| Entire | Name: SthK P300A bound to cAMP |
|---|---|
| Components |
|
-Supramolecule #1: SthK P300A bound to cAMP
| Supramolecule | Name: SthK P300A bound to cAMP / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 / Details: SthK P300A was reconstituted into nanodiscs. |
|---|---|
| Source (natural) | Organism:  Spirochaeta thermophila (strain ATCC 700085 / DSM 6578 / Z-1203) (bacteria) Spirochaeta thermophila (strain ATCC 700085 / DSM 6578 / Z-1203) (bacteria) |
-Macromolecule #1: SthK
| Macromolecule | Name: SthK / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Spirochaeta thermophila (strain ATCC 700085 / DSM 6578 / Z-1203) (bacteria) Spirochaeta thermophila (strain ATCC 700085 / DSM 6578 / Z-1203) (bacteria)Strain: ATCC 700085 / DSM 6578 / Z-1203 |
| Molecular weight | Theoretical: 51.092539 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAKDIGINSD PNSSSVDKLM KSSGVSNPTY TLVWKVWILA VTLYYAIRIP LTLVFPSLFS PLLPLDILAS LALIADIPLD LAFESRRTS GRKPTLLAPS RLPDLLAALP LDLLVFALHL PSPLSLLSLV RLLKLISVQR SATRILSYRI NPALLRLLSL V GFILLAAH ...String: MAKDIGINSD PNSSSVDKLM KSSGVSNPTY TLVWKVWILA VTLYYAIRIP LTLVFPSLFS PLLPLDILAS LALIADIPLD LAFESRRTS GRKPTLLAPS RLPDLLAALP LDLLVFALHL PSPLSLLSLV RLLKLISVQR SATRILSYRI NPALLRLLSL V GFILLAAH GIACGWMSLQ PPSENPAGTR YLSAFYWTIT TLTTIGYGDI TPSTPTQTVY TIVIELLGAA MYGLVIGNIA SL VSKLDAA KLLHRERVER VTAFLSYKRI SPELQRRIIE YFDYLWETRR GYEEREVLKE LPHPLRLAVA MEIHGDVIEK VAL FKGAGE EFIRDIILHL EPVIYGPGEY IIRAGEMGSD VYFINRGSVE VLSADEKTRY AILSEGQFFG EMALILRAPR TATV RARAF CDLYRLDKET FDRILSRYPE IAAQIQELAV RRKELESSGL VPRGSVKHHH H UniProtKB: Transcriptional regulator, Crp/Fnr family |
-Macromolecule #2: ADENOSINE-3',5'-CYCLIC-MONOPHOSPHATE
| Macromolecule | Name: ADENOSINE-3',5'-CYCLIC-MONOPHOSPHATE / type: ligand / ID: 2 / Number of copies: 4 / Formula: CMP |
|---|---|
| Molecular weight | Theoretical: 329.206 Da |
| Chemical component information |  ChemComp-CMP: |
-Macromolecule #3: (1R)-2-{[(S)-{[(2S)-2,3-dihydroxypropyl]oxy}(hydroxy)phosphoryl]o...
| Macromolecule | Name: (1R)-2-{[(S)-{[(2S)-2,3-dihydroxypropyl]oxy}(hydroxy)phosphoryl]oxy}-1-[(hexadecanoyloxy)methyl]ethyl (9Z)-octadec-9-enoate type: ligand / ID: 3 / Number of copies: 24 / Formula: PGW |
|---|---|
| Molecular weight | Theoretical: 749.007 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 7.5 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| |||||||||||||||
| Grid | Model: Quantifoil, UltrAuFoil, R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 50 sec. / Pretreatment - Atmosphere: AIR | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Spherical aberration corrector: Microscope was modified with a Cs corrector Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Digitization - Frames/image: 1-50 / Number grids imaged: 1 / Number real images: 2494 / Average exposure time: 10.0 sec. / Average electron dose: 71.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 45620 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 0.001 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|---|
| Output model |  PDB-6vxz: |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)