+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20969 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of a substrate-engaged Bam complex | |||||||||||||||
 Map data Map data | ||||||||||||||||
 Sample Sample |
| |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationBam protein complex / Gram-negative-bacterium-type cell outer membrane assembly / protein insertion into membrane / cell outer membrane / protein-macromolecule adaptor activity / cell adhesion / response to antibiotic / cell surface / identical protein binding / membrane Similarity search - Function | |||||||||||||||
| Biological species |   | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.1 Å | |||||||||||||||
 Authors Authors | Tomasek D / Rawson S / Lee J / Wzorek JS / Harrison SC / Li Z / Kahne D | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2020 Journal: Nature / Year: 2020Title: Structure of a nascent membrane protein as it folds on the BAM complex. Authors: David Tomasek / Shaun Rawson / James Lee / Joseph S Wzorek / Stephen C Harrison / Zongli Li / Daniel Kahne /  Abstract: Mitochondria, chloroplasts and Gram-negative bacteria are encased in a double layer of membranes. The outer membrane contains proteins with a β-barrel structure. β-Barrels are sheets of β-strands ...Mitochondria, chloroplasts and Gram-negative bacteria are encased in a double layer of membranes. The outer membrane contains proteins with a β-barrel structure. β-Barrels are sheets of β-strands wrapped into a cylinder, in which the first strand is hydrogen-bonded to the final strand. Conserved multi-subunit molecular machines fold and insert these proteins into the outer membrane. One subunit of the machines is itself a β-barrel protein that has a central role in folding other β-barrels. In Gram-negative bacteria, the β-barrel assembly machine (BAM) consists of the β-barrel protein BamA, and four lipoproteins. To understand how the BAM complex accelerates folding without using exogenous energy (for example, ATP), we trapped folding intermediates on this machine. Here we report the structure of the BAM complex of Escherichia coli folding BamA itself. The BamA catalyst forms an asymmetric hybrid β-barrel with the BamA substrate. The N-terminal edge of the BamA catalyst has an antiparallel hydrogen-bonded interface with the C-terminal edge of the BamA substrate, consistent with previous crosslinking studies; the other edges of the BamA catalyst and substrate are close to each other, but curl inward and do not pair. Six hydrogen bonds in a membrane environment make the interface between the two proteins very stable. This stability allows folding, but creates a high kinetic barrier to substrate release after folding has finished. Features at each end of the substrate overcome this barrier and promote release by stepwise exchange of hydrogen bonds. This mechanism of substrate-assisted product release explains how the BAM complex can stably associate with the substrate during folding and then turn over rapidly when folding is complete. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20969.map.gz emd_20969.map.gz | 10.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20969-v30.xml emd-20969-v30.xml emd-20969.xml emd-20969.xml | 20.1 KB 20.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_20969.png emd_20969.png | 138.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20969 http://ftp.pdbj.org/pub/emdb/structures/EMD-20969 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20969 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20969 | HTTPS FTP |
-Validation report
| Summary document |  emd_20969_validation.pdf.gz emd_20969_validation.pdf.gz | 330.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_20969_full_validation.pdf.gz emd_20969_full_validation.pdf.gz | 329.9 KB | Display | |
| Data in XML |  emd_20969_validation.xml.gz emd_20969_validation.xml.gz | 7 KB | Display | |
| Data in CIF |  emd_20969_validation.cif.gz emd_20969_validation.cif.gz | 7.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20969 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20969 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20969 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20969 | HTTPS FTP |
-Related structure data
| Related structure data |  6v05MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20969.map.gz / Format: CCP4 / Size: 184 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20969.map.gz / Format: CCP4 / Size: 184 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
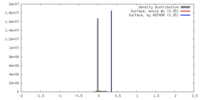
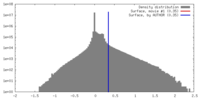
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : BamABCDE bound to substrate BamA with loop 1 deleted
| Entire | Name: BamABCDE bound to substrate BamA with loop 1 deleted |
|---|---|
| Components |
|
-Supramolecule #1: BamABCDE bound to substrate BamA with loop 1 deleted
| Supramolecule | Name: BamABCDE bound to substrate BamA with loop 1 deleted / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
-Macromolecule #1: Outer membrane protein assembly factor BamA
| Macromolecule | Name: Outer membrane protein assembly factor BamA / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 90.567227 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAMKKLLIAS LLFSSATVYG AEGFVVKDIH FEGLQRVAVG AALLSMPVRT GDTVNDEDIS NTIRALFATG NFEDVRVLRD GDTLLVQVK ERPTIASITF SGNKSVKDDM LKQNLEASGV RVGESLDRTT IADIEKGLED FYYSVGKYSA SVKAVVTPLP R NRVDLKLV ...String: MAMKKLLIAS LLFSSATVYG AEGFVVKDIH FEGLQRVAVG AALLSMPVRT GDTVNDEDIS NTIRALFATG NFEDVRVLRD GDTLLVQVK ERPTIASITF SGNKSVKDDM LKQNLEASGV RVGESLDRTT IADIEKGLED FYYSVGKYSA SVKAVVTPLP R NRVDLKLV FQEGVSAEIQ QINIVGNHAF TTDELISHFQ LRDEVPWWNV VGDRKYQKQK LAGDLETLRS YYLDRGYARF NI DSTQVSL TPDKKGIYVT VNITEGDQYK LSGVEVSGNL AGHSAEIEQL TKIEPGELYN GTKVTKMEDD IKKLLGRYGY AYP RVQSMP EINDADKTVK LRVNVDAGNR FYVRKIRFEG NDTSKDAVLR REMRQMEGAW LGSDLVDQGK ERLNRLGFFE TVDT DTQRV PGSPDQVDVV YKVKERNTGS FNFGIGYGTE SGVSFQAGVQ QDNWLGTGYA VGINGTKNDY QTYAELSVTN PYFTV DGVS LGGRLFYNDF QADDADLSDY TNKSYGTDVT LGFPINEYNS LRAGLGYVHN SLSNMQPQVA MWRYLYSMGE HPSTSD QDN SFKTDDFTFN YGWTYNKLDR GYFPTDGSRV NLTGKVTIPG SDNEYYKVTL DTATYVPIDD DHKWVVLGRT RWGYGDG LG GKEMPFYENF YAGGSSTVRG FQSNTIGPKA VYFPHQASNY DPDYDYESAT QDGAKDLSKS DDAVGGNAMA VASLEFIT P TPFISDKYAN SVRTSFFWDM GTVWDTNWDS SQYSGYPDYS DPSNIRMSAG IALQWMSPLG PLVFSYAQPF KKYDGDKAE QFQCNIGKTW |
-Macromolecule #2: Outer membrane protein assembly factor BamB
| Macromolecule | Name: Outer membrane protein assembly factor BamB / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 41.918945 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MQLRKLLLPG LLSVTLLSGC SLFNSEEDVV KMSPLPTVEN QFTPTTAWST SVGSGIGNFY SNLHPALADN VVYAADRAGL VKALNADDG KEIWSVSLAE KDGWFSKEPA LLSGGVTVSG GHVYIGSEKA QVYALNTSDG TVAWQTKVAG EALSRPVVSD G LVLIHTSN ...String: MQLRKLLLPG LLSVTLLSGC SLFNSEEDVV KMSPLPTVEN QFTPTTAWST SVGSGIGNFY SNLHPALADN VVYAADRAGL VKALNADDG KEIWSVSLAE KDGWFSKEPA LLSGGVTVSG GHVYIGSEKA QVYALNTSDG TVAWQTKVAG EALSRPVVSD G LVLIHTSN GQLQALNEAD GAVKWTVNLD MPSLSLRGES APTTAFGAAV VGGDNGRVSA VLMEQGQMIW QQRISQATGS TE IDRLSDV DTTPVVVNGV VFALAYNGNL TALDLRSGQI MWKRELGSVN DFIVDGNRIY LVDQNDRVMA LTIDGGVTLW TQS DLLHRL LTSPVLYNGN LVVGDSEGYL HWINVEDGRF VAQQKVDSSG FQTEPVAADG KLLIQAKDGT VYSITR |
-Macromolecule #3: Outer membrane protein assembly factor BamC
| Macromolecule | Name: Outer membrane protein assembly factor BamC / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 36.875277 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAYSVQKSRL AKVAGVSLVL LLAACSSDSR YKRQVSGDEA YLEAAPLAEL HAPAGMILPV TSGDYAIPVT NGSGAVGKAL DIRPPAQPL ALVSGARTQF TGDTASLLVE NGRGNTLWPQ VVSVLQAKNY TITQRDDAGQ TLTTDWVQWN RLDEDEQYRG R YQISVKPQ ...String: MAYSVQKSRL AKVAGVSLVL LLAACSSDSR YKRQVSGDEA YLEAAPLAEL HAPAGMILPV TSGDYAIPVT NGSGAVGKAL DIRPPAQPL ALVSGARTQF TGDTASLLVE NGRGNTLWPQ VVSVLQAKNY TITQRDDAGQ TLTTDWVQWN RLDEDEQYRG R YQISVKPQ GYQQAVTVKL LNLEQAGKPV ADAASMQRYS TEMMNVISAG LDKSATDAAN AAQNRASTTM DVQSAADDTG LP MLVVRGP FNVVWQRLPA ALEKVGMKVT DSTRSQGNMA VTYKPLSDSD WQELGASDPG LASGDYKLQV GDLDNRSSLQ FID PKGHTL TQSQNDALVA VFQAAFSK |
-Macromolecule #4: Outer membrane protein assembly factor BamD
| Macromolecule | Name: Outer membrane protein assembly factor BamD / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 27.85835 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTRMKYLVAA ATLSLFLAGC SGSKEEVPDN PPNEIYATAQ QKLQDGNWRQ AITQLEALDN RYPFGPYSQQ VQLDLIYAYY KNADLPLAQ AAIDRFIRLN PTHPNIDYVM YMRGLTNMAL DDSALQGFFG VDRSDRDPQH ARAAFSDFSK LVRGYPNSQY T TDATKRLV ...String: MTRMKYLVAA ATLSLFLAGC SGSKEEVPDN PPNEIYATAQ QKLQDGNWRQ AITQLEALDN RYPFGPYSQQ VQLDLIYAYY KNADLPLAQ AAIDRFIRLN PTHPNIDYVM YMRGLTNMAL DDSALQGFFG VDRSDRDPQH ARAAFSDFSK LVRGYPNSQY T TDATKRLV FLKDRLAKYE YSVAEYYTER GAWVAVVNRV EGMLRDYPDT QATRDALPLM ENAYRQMQMN AQAEKVAKII AA NSSNT |
-Macromolecule #5: Outer membrane protein assembly factor BamE
| Macromolecule | Name: Outer membrane protein assembly factor BamE / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 13.530256 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MRCKTLTAAA AVLLMLTAGC STLERVVYRP DINQGNYLTA NDVSKIRVGM TQQQVAYALG TPLMSDPFGT NTWFYVFRQQ PGHEGVTQQ TLTLTFNSSG VLTNIDNKPA LSGNGGHHHH HHHH |
-Macromolecule #6: Outer membrane protein assembly factor BamA,Outer membrane protei...
| Macromolecule | Name: Outer membrane protein assembly factor BamA,Outer membrane protein assembly factor BamA,Outer membrane protein assembly factor BamA,Outer membrane protein assembly factor BamA type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 64.010285 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAMKKLLIAS LLFSSATVYG AWSHPQFEKG GGSGGGSGGS AWSHPQFEKE GFVVKDIHFE GLQRVAVGAA LLSMPVRTGD TVNDEDISN TIRALFATGN FEDVRVLRDG DTLLVQVKER PTIASITFSG NKSVKDDMLK QNLEASGVRV GESLDRTTIA D IEKGLEDF ...String: MAMKKLLIAS LLFSSATVYG AWSHPQFEKG GGSGGGSGGS AWSHPQFEKE GFVVKDIHFE GLQRVAVGAA LLSMPVRTGD TVNDEDISN TIRALFATGN FEDVRVLRDG DTLLVQVKER PTIASITFSG NKSVKDDMLK QNLEASGVRV GESLDRTTIA D IEKGLEDF YYSVGKYSAS VKAVVTPLPR NRVDLKLVFQ ENTGSFNFGF QAGVQQDNWL GTGYAVGING TKNDYQCYAE LS VTNPYFT VDGVSLGGRL FYNDFQADDA DLSDYTNKSY GTDVTLGFPI NEYNSLRAGL GYVHNSLSNM QPQVAMWRYL YSM GEHPST SDQDNSFKTD DFTFNYGWTY NKLDRGYFPT DGSRVNLTGK VTIPGSDNEY YKVTLDTATY VPIDDDHKWV VLGR TRWGY GDGLGGKEMP FYENFYAGGS STVRGFQSNT IGPKAVYFPH QASNYDPDYD YESATQDGAK DLSKSDDAVG GNAMA VASL EFITPTPFIS DKYANSVRTS FFWDMGTVWD TNWDSSQYSG YPDYSDPSNI RMSAGIALQW MSPLGPLVFS YAQPFK KYD GDKAEQFQFN IGKTW |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 400 / Pretreatment - Type: GLOW DISCHARGE | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 298 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 25 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number real images: 4097 / Average exposure time: 3.0 sec. / Average electron dose: 70.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 2.8 µm / Calibrated defocus min: 1.1 µm / Calibrated magnification: 58717 / Illumination mode: OTHER / Imaging mode: OTHER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Particle selection | Number selected: 2054956 |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 4.1 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 223353 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)