[English] 日本語
 Yorodumi
Yorodumi- PDB-1qmg: Acetohydroxyacid isomeroreductase complexed with its reaction pro... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1qmg | ||||||
|---|---|---|---|---|---|---|---|
| Title | Acetohydroxyacid isomeroreductase complexed with its reaction product dihydroxy-methylvalerate, manganese and ADP-ribose. | ||||||
 Components Components | ACETOHYDROXY-ACID ISOMEROREDUCTASE | ||||||
 Keywords Keywords | OXIDOREDUCTASE / BRANCHED CHAIN AMINO ACID BIOSYNTHESIS / REACTION PRODUCT / MANGANESE / ADP-RIBOSE | ||||||
| Function / homology |  Function and homology information Function and homology informationketol-acid reductoisomerase (NADP+) / ketol-acid reductoisomerase activity / L-valine biosynthetic process / isoleucine biosynthetic process / NADPH binding / chloroplast / magnesium ion binding / protein homodimerization activity / mitochondrion Similarity search - Function | ||||||
| Biological species |  SPINACIA OLERACEA (spinach) SPINACIA OLERACEA (spinach) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.6 Å MOLECULAR REPLACEMENT / Resolution: 1.6 Å | ||||||
 Authors Authors | Thomazeau, K. / Dumas, R. / Halgand, F. / Douce, R. / Biou, V. | ||||||
 Citation Citation |  Journal: Acta Crystallogr.,Sect.D / Year: 2000 Journal: Acta Crystallogr.,Sect.D / Year: 2000Title: Structure of Spinach Acetohydroxyacid Isomeroreductase Complexed with its Product of Reaction Dihydroxy-Methylvalerate, Manganese and Adp-Ribose Authors: Thomazeau, K. / Dumas, R. / Halgand, F. / Forest, E. / Douce, R. / Biou, V. #1:  Journal: Embo J. / Year: 1997 Journal: Embo J. / Year: 1997Title: The Crystal Structure of Plant Acetohydroxyacid Isomeroreductase Complexed with its Reaction Product Dihydroxymethylvalerate, Manganese and (Phospho)-Adp-Ribose Authors: Biou, V. / Dumas, R. / Cohen-Addad, C. / Douce, R. / Job, D. / Pebay-Peyroula, E. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1qmg.cif.gz 1qmg.cif.gz | 442.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1qmg.ent.gz pdb1qmg.ent.gz | 360.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1qmg.json.gz 1qmg.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qm/1qmg https://data.pdbj.org/pub/pdb/validation_reports/qm/1qmg ftp://data.pdbj.org/pub/pdb/validation_reports/qm/1qmg ftp://data.pdbj.org/pub/pdb/validation_reports/qm/1qmg | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1yveS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||
| 2 | 
| ||||||||||||||||
| Unit cell |
| ||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS oper:
|
- Components
Components
-Protein , 1 types, 4 molecules ABCD
| #1: Protein | Mass: 57045.543 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  SPINACIA OLERACEA (spinach) / Organelle: PLASTID / Plasmid: PKK223.3 SPINACIA OLERACEA (spinach) / Organelle: PLASTID / Plasmid: PKK223.3Gene (production host): CDNA FROM ACETOHYDROXY ACID ISOMEROREDUCTASE Production host:  References: UniProt: Q01292, ketol-acid reductoisomerase (NADP+) |
|---|
-Non-polymers , 5 types, 2106 molecules 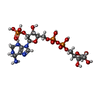


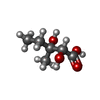





| #2: Chemical | ChemComp-APX / #3: Chemical | ChemComp-MN / #4: Chemical | ChemComp-SO4 / | #5: Chemical | ChemComp-DMV / #6: Water | ChemComp-HOH / | |
|---|
-Details
| Sequence details | THE MATURE AMINO ACID SEQUENCE IS DEVOID OF SIGNAL PEPTIDE. THEREFORE, RESIDUE 72 IS THE N-TERMINAL ...THE MATURE AMINO ACID SEQUENCE IS DEVOID OF SIGNAL PEPTIDE. THEREFORE, RESIDUE 72 IS THE N-TERMINAL AMINO ACID. |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.4 Å3/Da / Density % sol: 54 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 7.2 Details: PROTEIN WAS CRYSTALLISED FROM 1.8 M AMMONIUM SULFATE IN 0.1 M TRIS-HCL AT PH 7.2 IN THE PRESENCE OF AHB, NADPH, AND MN2+. | ||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 20 ℃ / pH: 7.5 / Method: vapor diffusion, hanging drop / Details: Dumas, R., (1994) J. Mol. Biol., 242, 578. | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: BM14 / Wavelength: 0.984 / Beamline: BM14 / Wavelength: 0.984 |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: Jun 15, 1996 / Details: 2 MIRRORS |
| Radiation | Monochromator: SI(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.984 Å / Relative weight: 1 |
| Reflection | Resolution: 1.6→26 Å / Num. obs: 299230 / % possible obs: 96.6 % / Redundancy: 2.9 % / Biso Wilson estimate: 14.1 Å2 / Rmerge(I) obs: 0.041 / Rsym value: 0.054 / Net I/σ(I): 18.8 |
| Reflection shell | Resolution: 1.6→1.64 Å / Redundancy: 2.9 % / Rmerge(I) obs: 0.079 / Mean I/σ(I) obs: 14.6 / Rsym value: 0.096 / % possible all: 96.6 |
| Reflection | *PLUS Rmerge(I) obs: 0.054 |
| Reflection shell | *PLUS % possible obs: 96.6 % / Rmerge(I) obs: 0.096 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1YVE Resolution: 1.6→10 Å / Rfactor Rfree error: 0.002 / Data cutoff high absF: 2603215.5 / Data cutoff low absF: 0 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 Details: NCS RESTRAINTS HAVE BEEN APPLIED UNTIL THE R-FACTOR DROPPED TO A VALUE OF 0.200. THEN THEY WERE PROGRESSIVELY LOOSENED AND REMOVED BECAUSE THE RATIO BETWEEN THE NUMBER OF REFLECTIONS AND ...Details: NCS RESTRAINTS HAVE BEEN APPLIED UNTIL THE R-FACTOR DROPPED TO A VALUE OF 0.200. THEN THEY WERE PROGRESSIVELY LOOSENED AND REMOVED BECAUSE THE RATIO BETWEEN THE NUMBER OF REFLECTIONS AND NUMBER OF DEGREES OF FREEDOM MADE IT POSSIBLE.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 16.6 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.6→10 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.6→1.7 Å / Rfactor Rfree error: 0.006 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.851 / Classification: refinement X-PLOR / Version: 3.851 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS σ(F): 0 / Rfactor obs: 0.193 / Rfactor Rfree: 0.225 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller












 PDBj
PDBj






