[English] 日本語
 Yorodumi
Yorodumi- PDB-1b5q: A 30 ANGSTROM U-SHAPED CATALYTIC TUNNEL IN THE CRYSTAL STRUCTURE ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1b5q | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | A 30 ANGSTROM U-SHAPED CATALYTIC TUNNEL IN THE CRYSTAL STRUCTURE OF POLYAMINE OXIDASE | ||||||||||||
 Components Components | PROTEIN (POLYAMINE OXIDASE) | ||||||||||||
 Keywords Keywords | OXIDOREDUCTASE / FLAVIN-DEPENDENT AMINE OXIDASE | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationpolyamine oxidase (propane-1,3-diamine-forming) / N8-acetylspermidine oxidase (propane-1,3-diamine-forming) / spermine oxidase (propane-1,3-diamine-forming) activity / N8-acetylspermidine:oxygen oxidoreductase (propane-1,3-diamine-forming) activity / polyamine oxidase activity / polyamine catabolic process / spermine catabolic process / plant-type cell wall / apoplast / flavin adenine dinucleotide binding Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MIR / Resolution: 1.9 Å MIR / Resolution: 1.9 Å | ||||||||||||
 Authors Authors | Binda, C. / Coda, A. / Angelini, R. / Federico, R. / Ascenzi, P. / Mattevi, A. | ||||||||||||
 Citation Citation |  Journal: Structure Fold.Des. / Year: 1999 Journal: Structure Fold.Des. / Year: 1999Title: A 30-angstrom-long U-shaped catalytic tunnel in the crystal structure of polyamine oxidase. Authors: Binda, C. / Coda, A. / Angelini, R. / Federico, R. / Ascenzi, P. / Mattevi, A. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1b5q.cif.gz 1b5q.cif.gz | 306.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1b5q.ent.gz pdb1b5q.ent.gz | 247.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1b5q.json.gz 1b5q.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1b5q_validation.pdf.gz 1b5q_validation.pdf.gz | 2.2 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1b5q_full_validation.pdf.gz 1b5q_full_validation.pdf.gz | 2.3 MB | Display | |
| Data in XML |  1b5q_validation.xml.gz 1b5q_validation.xml.gz | 68 KB | Display | |
| Data in CIF |  1b5q_validation.cif.gz 1b5q_validation.cif.gz | 90.4 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/b5/1b5q https://data.pdbj.org/pub/pdb/validation_reports/b5/1b5q ftp://data.pdbj.org/pub/pdb/validation_reports/b5/1b5q ftp://data.pdbj.org/pub/pdb/validation_reports/b5/1b5q | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| 2 | 
| ||||||||||||
| 3 | 
| ||||||||||||
| Unit cell |
| ||||||||||||
| Noncrystallographic symmetry (NCS) | NCS oper:
|
- Components
Components
-Protein , 1 types, 3 molecules ABC
| #1: Protein | Mass: 53698.457 Da / Num. of mol.: 3 / Fragment: FAD-BINDING DOMAIN / Source method: isolated from a natural source / Source: (natural)  |
|---|
-Sugars , 2 types, 3 molecules
| #2: Polysaccharide | Source method: isolated from a genetically manipulated source #3: Polysaccharide | alpha-D-mannopyranose-(1-6)-alpha-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1- ...alpha-D-mannopyranose-(1-6)-alpha-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-D-fucopyranose-(1-3)]2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source |
|---|
-Non-polymers , 3 types, 634 molecules 
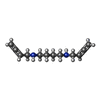



| #4: Chemical | | #5: Chemical | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4.3 Å3/Da / Density % sol: 71.37 % | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 4.6 / Details: pH 4.6 | ||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 293 K / pH: 6 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: BM14 / Wavelength: 1 / Beamline: BM14 / Wavelength: 1 |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.9→50 Å / Num. obs: 216570 / % possible obs: 98.1 % / Redundancy: 3.6 % / Rmerge(I) obs: 0.082 |
| Reflection | *PLUS Num. obs: 211025 / % possible obs: 96.6 % / Num. measured all: 704940 / Rmerge(I) obs: 0.088 |
| Reflection shell | *PLUS % possible obs: 95.2 % / Rmerge(I) obs: 0.173 / Mean I/σ(I) obs: 3.5 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MIR / Resolution: 1.9→50 Å / σ(F): 0 / Stereochemistry target values: TNT PROTGEO MIR / Resolution: 1.9→50 Å / σ(F): 0 / Stereochemistry target values: TNT PROTGEO
| ||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→50 Å
| ||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||
| Software | *PLUS Name: TNT / Classification: refinement | ||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 1.9 Å / Lowest resolution: 100 Å / σ(F): 0 / Num. reflection Rfree: 2125 / % reflection Rfree: 1 % / Rfactor all: 0.193 / Rfactor Rfree: 0.229 / Rfactor Rwork: 0.192 | ||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller










 PDBj
PDBj


