[English] 日本語
 Yorodumi
Yorodumi- EMDB-1569: Keyhole limpet hemocyanin (KLH): 9A cryoEM structure and molecula... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1569 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
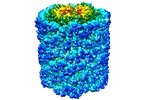
| Title | Keyhole limpet hemocyanin (KLH): 9A cryoEM structure and molecular model of the didecamer reveal the interfaces and intricate topology of the 160 functional units | |||||||||
 Map data Map data | Keyhole Limpet Hemocyanin Isoform 1 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | keyhole limpet hemocyanin / KLH / Gastropoda / Megathura crenulata / oxygen carrier | |||||||||
| Function / homology |  Function and homology information Function and homology informationoxygen carrier activity / oxidoreductase activity / extracellular region / metal ion binding Similarity search - Function | |||||||||
| Biological species |  Megathura crenulata (invertebrata) Megathura crenulata (invertebrata) | |||||||||
| Method | single particle reconstruction / cryo EM / negative staining / Resolution: 9.1 Å | |||||||||
 Authors Authors | Gatsogiannis C / Markl J | |||||||||
 Citation Citation |  Journal: J Mol Biol / Year: 2009 Journal: J Mol Biol / Year: 2009Title: Keyhole limpet hemocyanin: 9-A CryoEM structure and molecular model of the KLH1 didecamer reveal the interfaces and intricate topology of the 160 functional units. Authors: Christos Gatsogiannis / Jürgen Markl /  Abstract: Hemocyanins are blue copper-containing respiratory proteins in the hemolymph of many arthropods and molluscs. Molluscan hemocyanins are decamers, didecamers, or multidecamers of a 340- to 400-kDa ...Hemocyanins are blue copper-containing respiratory proteins in the hemolymph of many arthropods and molluscs. Molluscan hemocyanins are decamers, didecamers, or multidecamers of a 340- to 400-kDa polypeptide subunit containing seven or eight globular functional units (FUs; FU-a to FU-h), each with an oxygen-binding site. The decamers are short 35-nm hollow cylinders, with their lumen narrowed by a collar complex. Our recently published 9-A cryo-electron microscopy/crystal structure hybrid model of a 3.4-MDa cephalopod hemocyanin decamer [Nautilus pompilius hemocyanin (NpH)] revealed the pathway of the seven-FU subunit (340 kDa), 15 types of inter-FU interface, and an asymmetric collar consisting of five "arcs" (FU-g pairs). We now present a comparable hybrid model of an 8-MDa gastropod hemocyanin didecamer assembled from two asymmetric decamers [isoform keyhole limpet hemocyanin (KLH) 1 of the established immunogen KLH]. Compared to NpH, the KLH1 subunit (400 kDa) is C-terminally elongated by FU-h, which is further extended by a unique tail domain. We have found that the wall-and-arc structure of the KLH1 decamer is very similar to that of NpH. We have traced the subunit pathway and how it continues from KLH1-g to KLH1-h to form an annulus of five "slabs" (FU-h pairs) at one cylinder edge. The 15 types of inter-FU interface detected in NpH are also present in KLH1. Moreover, we have identified one arc/slab interface, two slab/slab interfaces, five slab/wall interfaces, and four decamer/decamer interfaces. The 27 interfaces are described on the basis of two subunit conformers, yielding an asymmetric homodimer. Six protrusions from the cryo-electron microscopy structure per subunit are associated with putative attachment sites for N-linked glycans, indicating a total of 120 sugar trees in KLH1. Also, putative binding sites for divalent cations have been detected. In conclusion, the present 9-A data on KLH1 confirm and substantially broaden our recent analysis of the smaller cephalopod hemocyanin and essentially solve the gastropod hemocyanin structure. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1569.map.gz emd_1569.map.gz | 54.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1569-v30.xml emd-1569-v30.xml emd-1569.xml emd-1569.xml | 8.8 KB 8.8 KB | Display Display |  EMDB header EMDB header |
| Images |  1569.png 1569.png | 860.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1569 http://ftp.pdbj.org/pub/emdb/structures/EMD-1569 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1569 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1569 | HTTPS FTP |
-Related structure data
| Related structure data |  4bedMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_1569.map.gz / Format: CCP4 / Size: 339.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1569.map.gz / Format: CCP4 / Size: 339.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Keyhole Limpet Hemocyanin Isoform 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.24 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Keyhole Limpet Hemocyanin Isoform 1 (KLH1)
| Entire | Name: Keyhole Limpet Hemocyanin Isoform 1 (KLH1) |
|---|---|
| Components |
|
-Supramolecule #1000: Keyhole Limpet Hemocyanin Isoform 1 (KLH1)
| Supramolecule | Name: Keyhole Limpet Hemocyanin Isoform 1 (KLH1) / type: sample / ID: 1000 Oligomeric state: KLH1 is a didecamer of a 400 kDa subunit. Each subunit is composed by 8 paralogous O2 binding functional units Number unique components: 1 |
|---|---|
| Molecular weight | Theoretical: 8 MDa |
-Macromolecule #1: Keyhole Limpet hemocyanin Isoform 1
| Macromolecule | Name: Keyhole Limpet hemocyanin Isoform 1 / type: protein_or_peptide / ID: 1 / Name.synonym: KLH1 / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:  Megathura crenulata (invertebrata) Megathura crenulata (invertebrata) |
| Molecular weight | Theoretical: 8 MDa |
-Experimental details
-Structure determination
| Method | negative staining, cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.7 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Details: 50 mM Tris-Hcl, 150 mM NaCl, 5mM CaCl, 5mM MgCl2 |
| Staining | Type: NEGATIVE / Details: cryoEM, no stain |
| Grid | Details: 400 mesh copper |
| Vitrification | Cryogen name: ETHANE / Chamber temperature: 86 K / Instrument: HOMEMADE PLUNGER Details: Vitrification instrument: Home made. Vitrification carried out in 25 percent oxygen atmosphere Method: Single side blotting and rapid plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI 20 |
|---|---|
| Temperature | Average: 86 K |
| Date | Jul 25, 2007 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: PRIMESCAN / Digitization - Sampling interval: 1.24 µm / Number real images: 98 / Bits/pixel: 8 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 50000 / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 50000 |
| Sample stage | Specimen holder: Gatan single-tilt cryoholde / Specimen holder model: GATAN LIQUID NITROGEN |
- Image processing
Image processing
| CTF correction | Details: CTFFIND3 and TRANSFER, IMAGIC 5 |
|---|---|
| Final reconstruction | Applied symmetry - Point group: D5 (2x5 fold dihedral) / Resolution.type: BY AUTHOR / Resolution: 9.1 Å / Resolution method: OTHER / Software - Name: IMAGIC-5 / Number images used: 4762 |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)