+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Solution BcsD structure | |||||||||
 Map data Map data | BcsD octamer in solution : Unsharpened cryo-EM map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Bacterial cytoskeleton / Crystalline cellulose secretion / STRUCTURAL PROTEIN | |||||||||
| Function / homology | Cellulose synthase operon protein D, bacterial / Cellulose synthase subunit D superfamily / Cellulose synthase subunit D / cellulose biosynthetic process / Cellulose biosynthesis protein Function and homology information Function and homology information | |||||||||
| Biological species |  Komagataeibacter hansenii ATCC 23769 (bacteria) Komagataeibacter hansenii ATCC 23769 (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Krasteva PV / Abidi W / Decossas M | |||||||||
| Funding support | European Union, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2022 Journal: Sci Adv / Year: 2022Title: Bacterial crystalline cellulose secretion via a supramolecular BcsHD scaffold. Authors: Wiem Abidi / Marion Decossas / Lucía Torres-Sánchez / Lucie Puygrenier / Sylvie Létoffé / Jean-Marc Ghigo / Petya V Krasteva /  Abstract: Cellulose, the most abundant biopolymer on Earth, is not only the predominant constituent of plants but also a key extracellular polysaccharide in the biofilms of many bacterial species. Depending on ...Cellulose, the most abundant biopolymer on Earth, is not only the predominant constituent of plants but also a key extracellular polysaccharide in the biofilms of many bacterial species. Depending on the producers, chemical modifications, and three-dimensional assemblies, bacterial cellulose (BC) can present diverse degrees of crystallinity. Highly ordered, or crystalline, cellulose presents great economical relevance due to its ever-growing number of biotechnological applications. Even if some acetic acid bacteria have long been identified as BC superproducers, the molecular mechanisms determining the secretion of crystalline versus amorphous cellulose remain largely unknown. Here, we present structural and mechanistic insights into the role of the accessory subunits BcsH (CcpAx) and BcsD (CesD) that determine crystalline BC secretion in the lineage. We show that oligomeric BcsH drives the assembly of BcsD into a supramolecular cytoskeletal scaffold that likely stabilizes the cellulose-extruding synthase nanoarrays through an unexpected inside-out mechanism for secretion system assembly. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15041.map.gz emd_15041.map.gz | 61.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15041-v30.xml emd-15041-v30.xml emd-15041.xml emd-15041.xml | 18.4 KB 18.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15041_fsc.xml emd_15041_fsc.xml | 11.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_15041.png emd_15041.png | 48.2 KB | ||
| Masks |  emd_15041_msk_1.map emd_15041_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-15041.cif.gz emd-15041.cif.gz | 5.8 KB | ||
| Others |  emd_15041_additional_1.map.gz emd_15041_additional_1.map.gz emd_15041_half_map_1.map.gz emd_15041_half_map_1.map.gz emd_15041_half_map_2.map.gz emd_15041_half_map_2.map.gz | 117.6 MB 115.6 MB 115.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15041 http://ftp.pdbj.org/pub/emdb/structures/EMD-15041 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15041 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15041 | HTTPS FTP |
-Validation report
| Summary document |  emd_15041_validation.pdf.gz emd_15041_validation.pdf.gz | 709.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_15041_full_validation.pdf.gz emd_15041_full_validation.pdf.gz | 708.7 KB | Display | |
| Data in XML |  emd_15041_validation.xml.gz emd_15041_validation.xml.gz | 18.9 KB | Display | |
| Data in CIF |  emd_15041_validation.cif.gz emd_15041_validation.cif.gz | 24.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15041 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15041 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15041 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15041 | HTTPS FTP |
-Related structure data
| Related structure data |  7zzyMC  7zzqC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_15041.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15041.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BcsD octamer in solution : Unsharpened cryo-EM map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.93 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_15041_msk_1.map emd_15041_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
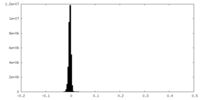
| Density Histograms |
-Additional map: BcsD octamer in solution : Autosharpened cryo-EM map (cryoSPARC)
| File | emd_15041_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BcsD octamer in solution : Autosharpened cryo-EM map (cryoSPARC) | ||||||||||||
| Projections & Slices |
| ||||||||||||
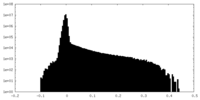
| Density Histograms |
-Half map: BcsD octamer in solution : half map A
| File | emd_15041_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BcsD octamer in solution : half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
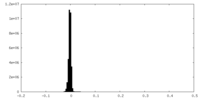
| Density Histograms |
-Half map: BcsD octamer in solution : half map B
| File | emd_15041_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BcsD octamer in solution : half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
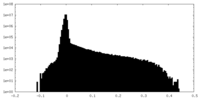
| Density Histograms |
- Sample components
Sample components
-Entire : BcsD from G. hansenii
| Entire | Name: BcsD from G. hansenii |
|---|---|
| Components |
|
-Supramolecule #1: BcsD from G. hansenii
| Supramolecule | Name: BcsD from G. hansenii / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: Octameric BcsD in solution. Expressed recombinantly in E. coli and purified via a cleavable N-terminal hexahistidine tag. |
|---|---|
| Source (natural) | Organism:  Komagataeibacter hansenii ATCC 23769 (bacteria) / Strain: ATCC 23769 Komagataeibacter hansenii ATCC 23769 (bacteria) / Strain: ATCC 23769 |
-Macromolecule #1: Cellulose biosynthesis protein
| Macromolecule | Name: Cellulose biosynthesis protein / type: protein_or_peptide / ID: 1 Details: The HRV3c-cleavable hexahistidine tag was cleaved during the purification process. Sample protein sequence starts with PMGSTIFEK... Number of copies: 8 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Komagataeibacter hansenii ATCC 23769 (bacteria) Komagataeibacter hansenii ATCC 23769 (bacteria) |
| Molecular weight | Theoretical: 20.596332 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSYYHHHHHH DYDIPTTLEV LFQGPMGSTI FEKKPDFTLF LQTLSWEIDD QVGIEVRNEL LREVGRGMGT RIMPPPCQTV DKLQIELNA LLALIGWGTV TLELLSEDQS LRIVHENLPQ VGSAGEPSGT WLAPVLEGLY GRWVTSQAGA FGDYVVTRDV D AEDLNAVP RQTIIMYMRV RSSAT UniProtKB: Cellulose biosynthesis protein |
-Macromolecule #2: water
| Macromolecule | Name: water / type: ligand / ID: 2 / Number of copies: 24 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.8 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: 20 mM HEPES pH 8.0, 100 mM NaCl |
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 40.59 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.75 µm / Nominal defocus min: 0.48 µm |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)