[English] 日本語
 Yorodumi
Yorodumi- EMDB-1442: The role of the kinesin-13 neck in microtubule depolymerization. -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1442 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The role of the kinesin-13 neck in microtubule depolymerization. | |||||||||
 Map data Map data | A realspace map of kinesin-13-bound, 15 protofilament microtubules, calculated from averaged layer line data using Fourier-Bessel synthesis | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 20.0 Å | |||||||||
 Authors Authors | Moores CA / Hekmat-Nejad M / Sakowicz R / Milligan RA | |||||||||
 Citation Citation |  Journal: Cell Cycle / Year: 2006 Journal: Cell Cycle / Year: 2006Title: The role of the kinesin-13 neck in microtubule depolymerization. Authors: Carolyn A Moores / Jeremy Cooper / Mike Wagenbach / Yulia Ovechkina / Linda Wordeman / Ronald A Milligan /  Abstract: To ensure genetic integrity, replicated chromosomes must be accurately distributed to daughter cells-a process that is accomplished on the microtubule spindle. Kinesin-13 motors play an essential ...To ensure genetic integrity, replicated chromosomes must be accurately distributed to daughter cells-a process that is accomplished on the microtubule spindle. Kinesin-13 motors play an essential role in this process by performing regulated microtubule depolymerization. We set out to dissect the depolymerization mechanism of these kinesins, and in particular, the role of their conserved neck sequence. We used a monomeric kinesin-13 MCAK, consisting of the neck and motor core, which has strong depolymerizing activity. In the presence of a non-hydrolysable ATP analogue, this construct induced formation of rings around microtubules. The rings are built from tubulin protofilaments that are bent by the kinesin-13 motor engaged at the ATP-binding step of its ATPase cycle. Our data suggest that the ring-microtubule interaction is mediated by the neck and support the idea of a role for the kinesin-13 neck in depolymerization efficiency, acting by optimizing release of tubulin from microtubule ends. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1442.map.gz emd_1442.map.gz | 476.5 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1442-v30.xml emd-1442-v30.xml emd-1442.xml emd-1442.xml | 10.4 KB 10.4 KB | Display Display |  EMDB header EMDB header |
| Images |  1442.gif 1442.gif | 109.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1442 http://ftp.pdbj.org/pub/emdb/structures/EMD-1442 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1442 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1442 | HTTPS FTP |
-Validation report
| Summary document |  emd_1442_validation.pdf.gz emd_1442_validation.pdf.gz | 274.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_1442_full_validation.pdf.gz emd_1442_full_validation.pdf.gz | 273.8 KB | Display | |
| Data in XML |  emd_1442_validation.xml.gz emd_1442_validation.xml.gz | 3.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1442 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1442 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1442 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1442 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_1442.map.gz / Format: CCP4 / Size: 2.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1442.map.gz / Format: CCP4 / Size: 2.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | A realspace map of kinesin-13-bound, 15 protofilament microtubules, calculated from averaged layer line data using Fourier-Bessel synthesis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.97 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
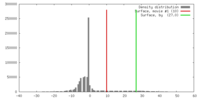
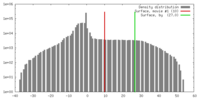
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Malarial kinesin-13 motor core ADPAlF
| Entire | Name: Malarial kinesin-13 motor core ADPAlF |
|---|---|
| Components |
|
-Supramolecule #1000: Malarial kinesin-13 motor core ADPAlF
| Supramolecule | Name: Malarial kinesin-13 motor core ADPAlF / type: sample / ID: 1000 / Details: Molecular weight of kinesin motor core is 40kD Oligomeric state: monomeric kinesin binds to each tubulin dimer Number unique components: 2 |
|---|
-Macromolecule #1: kinesin-13 motor core
| Macromolecule | Name: kinesin-13 motor core / type: protein_or_peptide / ID: 1 / Name.synonym: pKinI / Oligomeric state: monomer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  synonym: Malaria |
| Molecular weight | Experimental: 40 KDa / Theoretical: 40 KDa |
| Recombinant expression | Organism:  |
-Macromolecule #2: tubulin
| Macromolecule | Name: tubulin / type: protein_or_peptide / ID: 2 / Oligomeric state: helical polymer / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:  synonym: Malaria / Tissue: brain / Cell: neurone / Location in cell: cytoplasmic |
| Molecular weight | Experimental: 100 KDa / Theoretical: 100 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 3 mg/mL |
|---|---|
| Buffer | pH: 6.8 Details: 2mM ADP, 8mM NaF, 2mM AlCl3, 20mM Pipes, 2mM MgCl2, 1mM EGTA, 1mM DTT |
| Grid | Details: 400 mesh quantifoil grid 2/2 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 98 % / Chamber temperature: 277 K / Instrument: HOMEMADE PLUNGER Details: Vitrification instrument: plunger. vitrification performed in humidified cold room Method: blot for 3-4s before plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM200FEG |
|---|---|
| Temperature | Average: 95 K |
| Alignment procedure | Legacy - Astigmatism: objective lens astigmatism was corrected at |
| Date | Aug 1, 2002 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: PERKIN ELMER / Digitization - Sampling interval: 20 µm / Number real images: 50 / Average electron dose: 10 e/Å2 |
| Electron beam | Acceleration voltage: 120 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.95 µm / Nominal magnification: 38000 |
| Sample stage | Specimen holder: side entry, nitrogen cooled / Specimen holder model: GATAN LIQUID NITROGEN |
- Image processing
Image processing
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 20.0 Å / Resolution method: OTHER / Software - Name: Phoelix / Details: Final map calculated from 53 moire repeats |
|---|---|
| CTF correction | Details: each microtubule |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)