[English] 日本語
 Yorodumi
Yorodumi- EMDB-12178: Staphylococcus aureus 30S ribosomal subunit in presence of spermi... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12178 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Staphylococcus aureus 30S ribosomal subunit in presence of spermidine (body only) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Pathogen / small ribosomal subunit / spermidine / RIBOSOME | |||||||||
| Function / homology |  Function and homology information Function and homology informationribosomal small subunit biogenesis / small ribosomal subunit / small ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / tRNA binding / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex ...ribosomal small subunit biogenesis / small ribosomal subunit / small ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / tRNA binding / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Staphylococcus aureus subsp. aureus NCTC 8325 (bacteria) / Staphylococcus aureus subsp. aureus NCTC 8325 (bacteria) /   Staphylococcus aureus (strain NCTC 8325) (bacteria) Staphylococcus aureus (strain NCTC 8325) (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Belinite M / Khusainov I | |||||||||
| Funding support |  France, 2 items France, 2 items
| |||||||||
 Citation Citation |  Journal: Front Mol Biosci / Year: 2021 Journal: Front Mol Biosci / Year: 2021Title: Stabilization of Ribosomal RNA of the Small Subunit by Spermidine in . Authors: Margarita Belinite / Iskander Khusainov / Heddy Soufari / Stefano Marzi / Pascale Romby / Marat Yusupov / Yaser Hashem /   Abstract: Cryo-electron microscopy is now used as a method of choice in structural biology for studying protein synthesis, a process mediated by the ribosome machinery. In order to achieve high-resolution ...Cryo-electron microscopy is now used as a method of choice in structural biology for studying protein synthesis, a process mediated by the ribosome machinery. In order to achieve high-resolution structures using this approach, one needs to obtain homogeneous and stable samples, which requires optimization of ribosome purification in a species-dependent manner. This is especially critical for the bacterial small ribosomal subunit that tends to be unstable in the absence of ligands. Here, we report a protocol for purification of stable 30 S from the Gram-positive bacterium and its cryo-EM structures: in presence of spermidine at a resolution ranging between 3.4 and 3.6 Å and in its absence at 5.3 Å. Using biochemical characterization and cryo-EM, we demonstrate the importance of spermidine for stabilization of the 30 S preserving favorable conformation of the helix 44. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12178.map.gz emd_12178.map.gz | 6.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12178-v30.xml emd-12178-v30.xml emd-12178.xml emd-12178.xml | 25.9 KB 25.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_12178.png emd_12178.png | 75.4 KB | ||
| Filedesc metadata |  emd-12178.cif.gz emd-12178.cif.gz | 7.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12178 http://ftp.pdbj.org/pub/emdb/structures/EMD-12178 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12178 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12178 | HTTPS FTP |
-Related structure data
| Related structure data |  7bgdMC  7bgeC  7kwgC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12178.map.gz / Format: CCP4 / Size: 75.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12178.map.gz / Format: CCP4 / Size: 75.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.19 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
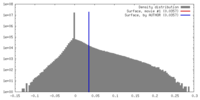
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Staphylococcus aureus 30S ribosomal subunit in the presence of sp...
+Supramolecule #1: Staphylococcus aureus 30S ribosomal subunit in the presence of sp...
+Supramolecule #2: 30S ribosomal subunit
+Supramolecule #3: mRNA
+Macromolecule #1: mRNA
+Macromolecule #2: 16S ribosomal RNA
+Macromolecule #3: 30S ribosomal protein S2
+Macromolecule #4: 30S ribosomal protein S4
+Macromolecule #5: 30S ribosomal protein S5
+Macromolecule #6: 30S ribosomal protein S6
+Macromolecule #7: 30S ribosomal protein S8
+Macromolecule #8: 30S ribosomal protein S11
+Macromolecule #9: 30S ribosomal protein S12
+Macromolecule #10: 30S ribosomal protein S15
+Macromolecule #11: 30S ribosomal protein S16
+Macromolecule #12: 30S ribosomal protein S17
+Macromolecule #13: 30S ribosomal protein S18
+Macromolecule #14: 30S ribosomal protein S20
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Average electron dose: 3.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 529602 |
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: PROJECTION MATCHING |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)