+Search query
-Structure paper
| Title | Ligand binding to a Ni-Fe cluster orchestrates conformational changes of the CO-dehydrogenase-acetyl-CoA synthase complex. |
|---|---|
| Journal, issue, pages | Nat Catal, Vol. 8, Issue 7, Page 657-667, Year 2025 |
| Publish date | Jul 11, 2025 |
 Authors Authors | Jakob Ruickoldt / Julian Kreibich / Thomas Bick / Jae-Hun Jeoung / Benjamin R Duffus / Silke Leimkühler / Holger Dobbek / Petra Wendler /  |
| PubMed Abstract | Catalytic metal clusters play critical roles in important enzymatic pathways such as carbon fixation and energy conservation. However, how ligand binding to the active-site metal regulates ...Catalytic metal clusters play critical roles in important enzymatic pathways such as carbon fixation and energy conservation. However, how ligand binding to the active-site metal regulates conformational changes critical for enzyme function is often not well understood. One carbon fixation pathway that relies heavily on metalloenzymes is the reductive acetyl-coenzyme A (acetyl-CoA) pathway. In this study, we investigated the catalysis of the last step of the reductive acetyl-CoA pathway by the CO-dehydrogenase (CODH)-acetyl-CoA synthase (ACS) complex from , focusing on how ligand binding to the nickel atom in the active site affects the conformational equilibrium of the enzyme. We captured six intermediate states of the enzyme by cryo-electron microscopy, with resolutions of 2.5-1.9 Å, and visualized reaction products bound to cluster A (an Ni,Ni-[4Fe4S] cluster) and identified several previously uncharacterized conformational states of CODH-ACS. The structures demonstrate how substrate binding controls conformational changes in the ACS subunit to prepare for the next catalytic step. |
 External links External links |  Nat Catal / Nat Catal /  PubMed:40727002 / PubMed:40727002 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 1.9 - 10.0 Å |
| Structure data | EMDB-50588, PDB-9fnc: EMDB-50598, PDB-9fnj: EMDB-50616, PDB-9fo4: EMDB-50626, PDB-9fop: EMDB-50631, PDB-9fox: EMDB-50674, PDB-9fr0: EMDB-50677, PDB-9fr1:  EMDB-50729: Extended state of carbonylated CODH/ACS (3D flex consensus map) EMDB-50754, PDB-9fu3: EMDB-50756, PDB-9fu4: EMDB-50757, PDB-9fu7: EMDB-50758, PDB-9fu9: EMDB-50759, PDB-9fua: EMDB-50760, PDB-9fub: EMDB-50761, PDB-9fuc:  EMDB-50837: CODH/ACS in the loose extended state in the presence of CoA (3D flex consensus map) |
| Chemicals |  ChemComp-RQM:  ChemComp-SF4:  ChemComp-CMO:  ChemComp-NI:  ChemComp-NA:  ChemComp-HOH:  ChemComp-FES:  ChemComp-ACE: 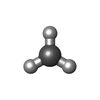 ChemComp-74C: |
| Source |
|
 Keywords Keywords | OXIDOREDUCTASE / CODH / ACS / CO2 fixation / reductive acetyl-CoA pathway / Wood-Ljungdahl pathway |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers
































 carboxydothermus hydrogenoformans (bacteria)
carboxydothermus hydrogenoformans (bacteria)