+Search query
-Structure paper
| Title | Structural basis for substrate binding, catalysis, and inhibition of cancer target mitochondrial creatine kinase by a covalent inhibitor. |
|---|---|
| Journal, issue, pages | Structure, Vol. 33, Issue 4, Page 786-797.e3, Year 2025 |
| Publish date | Apr 3, 2025 |
 Authors Authors | Merve Demir / Laura Koepping / Ya Li / Lynn Fujimoto / Andrey Bobkov / Jianhua Zhao / Taro Hitosugi / Eduard Sergienko /  |
| PubMed Abstract | Mitochondrial creatine kinases (MtCKs) are key players in maintaining energy homeostasis in cells that work with cytosolic creatine kinases for energy transport from mitochondria to cytoplasm. The ...Mitochondrial creatine kinases (MtCKs) are key players in maintaining energy homeostasis in cells that work with cytosolic creatine kinases for energy transport from mitochondria to cytoplasm. The inhibition of breast cancer growth by cyclocreatine targeting CKs indicates dependence of cancer cells on the "energy shuttle" for cell growth and survival. Hence, understanding key mechanistic features of creatine kinases and their inhibition plays an important role in the development of cancer therapeutics. Herein, we present mutational and structural investigations on understudied ubiquitous MtCK that showed closure of the loop comprising His61 is specific to and relies on creatine binding and mechanism of phosphoryl transfer depends on electrostatics of active site. We demonstrate that previously identified pan-CK covalent inhibitor CKi inhibit breast cancer cell proliferation; however, our biochemical and structural data indicated that inhibition by CKi is highly dependent on covalent link formation and conformational changes upon creatine binding are not observed. |
 External links External links |  Structure / Structure /  PubMed:39904336 / PubMed:39904336 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.2 - 2.89 Å |
| Structure data | EMDB-44028, PDB-9b04: EMDB-44029, PDB-9b05: EMDB-44055, PDB-9b0t: EMDB-44058, PDB-9b0u: EMDB-44068, PDB-9b14: EMDB-44069, PDB-9b16: |
| Chemicals |  ChemComp-ADP:  ChemComp-MG: 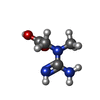 ChemComp-CRN:  ChemComp-NO3:  ChemComp-PO4: 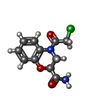 ChemComp-KLU: |
| Source |
|
 Keywords Keywords | TRANSFERASE / mitochondrial creatine kinase / TRANSFERASE/INHIBITOR / TRANSFERASE-INHIBITOR complex |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers















 homo sapiens (human)
homo sapiens (human)