+検索条件
-Structure paper
| タイトル | Structural basis of a microbial trimethylamine transporter. |
|---|---|
| ジャーナル・号・ページ | mBio, Vol. 16, Issue 1, Page e0191424, Year 2025 |
| 掲載日 | 2025年1月8日 |
 著者 著者 | Chao Gao / Hai-Tao Ding / Kang Li / Hai-Yan Cao / Ning Wang / Zeng-Tian Gu / Qing Wang / Mei-Ling Sun / Xiu-Lan Chen / Yin Chen / Yu-Zhong Zhang / Hui-Hui Fu / Chun-Yang Li /   |
| PubMed 要旨 | Trimethylamine (TMA), a simple trace biogenic amine resulting from the decomposition of proteins and other macromolecules, is ubiquitous in nature. It is found in the human gut as well as in various ...Trimethylamine (TMA), a simple trace biogenic amine resulting from the decomposition of proteins and other macromolecules, is ubiquitous in nature. It is found in the human gut as well as in various terrestrial and marine ecosystems. While the role of TMA in promoting cardiovascular diseases and depolarizing olfactory sensory neurons in humans has only recently been explored, many microbes are well known for their ability to utilize TMA as a carbon, nitrogen, and energy source. Here, we report the first structure of a TMA transporter, TmaT, originally identified from a marine bacterium. TmaT is a member of the betaine-choline-carnitine transporter family, and we show that TmaT is an Na/TMA symporter, which possessed high specificity and binding affinity toward TMA. Furthermore, the structures of TmaT and two TmaT-TMA complexes were solved by cryo-EM. TmaT forms a homotrimer structure in solution. Each TmaT monomer has 12 transmembrane helices, and the TMA transport channel is formed by a four-helix bundle. TMA can move between different aromatic boxes, which provides the structural basis of TmaT importing TMA. When TMA is bound in location I, residues Trp146, Trp151, Tyr154, and Trp326 form an aromatic box to accommodate TMA. Moreover, Met105 also plays an important role in the binding of TMA. When TMA is transferred to location II, it is bound in the aromatic box formed by Trp325, Trp326, and Trp329. Based on our results, we proposed the TMA transport mechanism by TmaT. This study provides novel insights into TMA transport across biological membranes. IMPORTANCE: The volatile trimethylamine (TMA) plays an important role in promoting cardiovascular diseases and depolarizing olfactory sensory neurons in humans and serves as a key nutrient source for a variety of ubiquitous marine microbes. While the TMA transporter TmaT has been identified from a marine bacterium, the structure of TmaT and the molecular mechanism involved in TMA transport remain unclear. In this study, we elucidated the high-resolution cryo-EM structures of TmaT and TmaT-TMA complexes and revealed the TMA binding and transport mechanisms by structural and biochemical analyses. The results advance our understanding of the TMA transport processes across biological membranes. |
 リンク リンク |  mBio / mBio /  PubMed:39576113 / PubMed:39576113 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) |
| 解像度 | 2.76 - 3.09 Å |
| 構造データ | EMDB-60519, PDB-8zw8: EMDB-60542, PDB-8zxk: EMDB-60548, PDB-8zxp: |
| 化合物 | 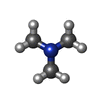 ChemComp-KEN: |
| 由来 |
|
 キーワード キーワード | ELECTRON TRANSPORT / TMA |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について









 myroides profundi (バクテリア)
myroides profundi (バクテリア)