+Search query
-Structure paper
| Title | Antigenic Characterization of the HCMV gH/gL/gO and Pentamer Cell Entry Complexes Reveals Binding Sites for Potently Neutralizing Human Antibodies. |
|---|---|
| Journal, issue, pages | PLoS Pathog, Vol. 11, Issue 10, Page e1005230, Year 2015 |
| Publish date | Oct 20, 2015 |
 Authors Authors | Claudio Ciferri / Sumana Chandramouli / Alexander Leitner / Danilo Donnarumma / Michael A Cianfrocco / Rachel Gerrein / Kristian Friedrich / Yukti Aggarwal / Giuseppe Palladino / Ruedi Aebersold / Nathalie Norais / Ethan C Settembre / Andrea Carfi /    |
| PubMed Abstract | Human Cytomegalovirus (HCMV) is a major cause of morbidity and mortality in transplant patients and in fetuses following congenital infection. The glycoprotein complexes gH/gL/gO and ...Human Cytomegalovirus (HCMV) is a major cause of morbidity and mortality in transplant patients and in fetuses following congenital infection. The glycoprotein complexes gH/gL/gO and gH/gL/UL128/UL130/UL131A (Pentamer) are required for HCMV entry in fibroblasts and endothelial/epithelial cells, respectively, and are targeted by potently neutralizing antibodies in the infected host. Using purified soluble forms of gH/gL/gO and Pentamer as well as a panel of naturally elicited human monoclonal antibodies, we determined the location of key neutralizing epitopes on the gH/gL/gO and Pentamer surfaces. Mass Spectrometry (MS) coupled to Chemical Crosslinking or to Hydrogen Deuterium Exchange was used to define residues that are either in proximity or part of neutralizing epitopes on the glycoprotein complexes. We also determined the molecular architecture of the gH/gL/gO- and Pentamer-antibody complexes by Electron Microscopy (EM) and 3D reconstructions. The EM analysis revealed that the Pentamer specific neutralizing antibodies bind to two opposite surfaces of the complex, suggesting that they may neutralize infection by different mechanisms. Together, our data identify the location of neutralizing antibodies binding sites on the gH/gL/gO and Pentamer complexes and provide a framework for the development of antibodies and vaccines against HCMV. |
 External links External links |  PLoS Pathog / PLoS Pathog /  PubMed:26485028 / PubMed:26485028 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 18.0 - 30.0 Å |
| Structure data |  EMDB-6431: 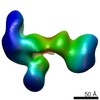 EMDB-6432: 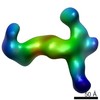 EMDB-6436: 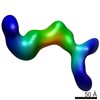 EMDB-6437:  EMDB-6438: |
| Source |
|
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers



 Cytomegalovirus
Cytomegalovirus