+Search query
-Structure paper
| Title | Structure, mechanism, and inhibition of Hedgehog acyltransferase. |
|---|---|
| Journal, issue, pages | Mol Cell, Vol. 81, Issue 24, Page 5025-5038.e10, Year 2021 |
| Publish date | Dec 16, 2021 |
 Authors Authors | Claire E Coupland / Sebastian A Andrei / T Bertie Ansell / Loic Carrique / Pramod Kumar / Lea Sefer / Rebekka A Schwab / Eamon F X Byrne / Els Pardon / Jan Steyaert / Anthony I Magee / Thomas Lanyon-Hogg / Mark S P Sansom / Edward W Tate / Christian Siebold /   |
| PubMed Abstract | The Sonic Hedgehog (SHH) morphogen pathway is fundamental for embryonic development and stem cell maintenance and is implicated in various cancers. A key step in signaling is transfer of a palmitate ...The Sonic Hedgehog (SHH) morphogen pathway is fundamental for embryonic development and stem cell maintenance and is implicated in various cancers. A key step in signaling is transfer of a palmitate group to the SHH N terminus, catalyzed by the multi-pass transmembrane enzyme Hedgehog acyltransferase (HHAT). We present the high-resolution cryo-EM structure of HHAT bound to substrate analog palmityl-coenzyme A and a SHH-mimetic megabody, revealing a heme group bound to HHAT that is essential for HHAT function. A structure of HHAT bound to potent small-molecule inhibitor IMP-1575 revealed conformational changes in the active site that occlude substrate binding. Our multidisciplinary analysis provides a detailed view of the mechanism by which HHAT adapts the membrane environment to transfer an acyl chain across the endoplasmic reticulum membrane. This structure of a membrane-bound O-acyltransferase (MBOAT) superfamily member provides a blueprint for other protein-substrate MBOATs and a template for future drug discovery. |
 External links External links |  Mol Cell / Mol Cell /  PubMed:34890564 / PubMed:34890564 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.61 - 3.59 Å |
| Structure data | EMDB-13764, PDB-7q1u:  EMDB-13841:  EMDB-13842: EMDB-13860, PDB-7q6z:  EMDB-14578: Structure of Hedgehog acyltransferase (HHAT) in complex with megabody 177 bound to non-hydrolysable palmitoyl-CoA (Consensus Map) |
| Chemicals |  ChemComp-CLR:  ChemComp-HEM:  ChemComp-HD6:  ChemComp-MG:  ChemComp-PLM: 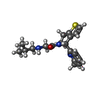 ChemComp-9V3: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN / HHAT / inhibitor / palmitoyl-CoA / co enzyme A / Hedgehog acyl transferase / Sonic Hedgehog / SHH / MBOAT / morphogen / palmitoylation / signalling / endoplasmic reticulum / heme / small molecule binding / drug target |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers








 homo sapiens (human)
homo sapiens (human)