+Search query
-Structure paper
| Title | Structural basis of CXC chemokine receptor 2 activation and signalling. |
|---|---|
| Journal, issue, pages | Nature, Vol. 585, Issue 7823, Page 135-140, Year 2020 |
| Publish date | Jul 1, 2020 |
 Authors Authors | Kaiwen Liu / Lijie Wu / Shuguang Yuan / Meng Wu / Yueming Xu / Qianqian Sun / Shu Li / Suwen Zhao / Tian Hua / Zhi-Jie Liu /  |
| PubMed Abstract | Chemokines and their receptors mediate cell migration, which influences multiple fundamental biological processes and disease conditions such as inflammation and cancer. Although ample effort has ...Chemokines and their receptors mediate cell migration, which influences multiple fundamental biological processes and disease conditions such as inflammation and cancer. Although ample effort has been invested into the structural investigation of the chemokine receptors and receptor-chemokine recognition, less is known about endogenous chemokine-induced receptor activation and G-protein coupling. Here we present the cryo-electron microscopy structures of interleukin-8 (IL-8, also known as CXCL8)-activated human CXC chemokine receptor 2 (CXCR2) in complex with G protein, along with a crystal structure of CXCR2 bound to a designed allosteric antagonist. Our results reveal a unique shallow mode of binding between CXCL8 and CXCR2, and also show the interactions between CXCR2 and G protein. Further structural analysis of the inactive and active states of CXCR2 reveals a distinct activation process and the competitive small-molecule antagonism of chemokine receptors. In addition, our results provide insights into how a G-protein-coupled receptor is activated by an endogenous protein molecule, which will assist in the rational development of therapeutics that target the chemokine system for better pharmacological profiles. |
 External links External links |  Nature / Nature /  PubMed:32610344 PubMed:32610344 |
| Methods | EM (single particle) / X-ray diffraction |
| Resolution | 3.2 - 3.5 Å |
| Structure data | EMDB-0877, PDB-6lfm: EMDB-0879, PDB-6lfo:  PDB-6lfl: |
| Chemicals | 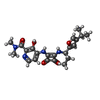 ChemComp-EBX:  ChemComp-CLR: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN / G protein-coupled receptor / G protein coupled-receptor |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers







 homo sapiens (human)
homo sapiens (human)
 pyrococcus abyssi (strain ge5 / orsay) (archaea)
pyrococcus abyssi (strain ge5 / orsay) (archaea)