+検索条件
-Structure paper
| タイトル | Structural basis of microRNA biogenesis by Dicer-1 and its partner protein Loqs-PB. |
|---|---|
| ジャーナル・号・ページ | Mol Cell, Vol. 82, Issue 21, Page 4049-44063.e6, Year 2022 |
| 掲載日 | 2022年11月3日 |
 著者 著者 | Karina Jouravleva / Dmitrij Golovenko / Gabriel Demo / Robert C Dutcher / Traci M Tanaka Hall / Phillip D Zamore / Andrei A Korostelev /   |
| PubMed 要旨 | In animals and plants, Dicer enzymes collaborate with double-stranded RNA-binding domain (dsRBD) proteins to convert precursor-microRNAs (pre-miRNAs) into miRNA duplexes. We report six cryo-EM ...In animals and plants, Dicer enzymes collaborate with double-stranded RNA-binding domain (dsRBD) proteins to convert precursor-microRNAs (pre-miRNAs) into miRNA duplexes. We report six cryo-EM structures of Drosophila Dicer-1 that show how Dicer-1 and its partner Loqs‑PB cooperate (1) before binding pre-miRNA, (2) after binding and in a catalytically competent state, (3) after nicking one arm of the pre-miRNA, and (4) following complete dicing and initial product release. Our reconstructions suggest that pre-miRNA binds a rare, open conformation of the Dicer‑1⋅Loqs‑PB heterodimer. The Dicer-1 dsRBD and three Loqs‑PB dsRBDs form a tight belt around the pre-miRNA, distorting the RNA helix to place the scissile phosphodiester bonds in the RNase III active sites. Pre-miRNA cleavage shifts the dsRBDs and partially closes Dicer-1, which may promote product release. Our data suggest a model for how the Dicer‑1⋅Loqs‑PB complex affects a complete cycle of pre-miRNA recognition, stepwise endonuclease cleavage, and product release. |
 リンク リンク |  Mol Cell / Mol Cell /  PubMed:36182693 / PubMed:36182693 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) |
| 解像度 | 3.06 - 4.02 Å |
| 構造データ | EMDB-27415, PDB-8dfv: EMDB-27416, PDB-8dg5: EMDB-27417, PDB-8dg7: EMDB-27420, PDB-8dga: EMDB-27423, PDB-8dgi: EMDB-27427, PDB-8dgj: |
| 化合物 |  ChemComp-CA:  ChemComp-MG: 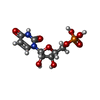 ChemComp-U5P: |
| 由来 |
|
 キーワード キーワード | RNA BINDING PROTEIN/RNA / Dicer / Dcr-1 / Loquacious / Loqs-P / miRNA / RNA BINDING PROTEIN-RNA complex / Loqs-PB |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について
















