+検索条件
-Structure paper
| タイトル | Subunit organization of the membrane-bound HIV-1 envelope glycoprotein trimer. |
|---|---|
| ジャーナル・号・ページ | Nat Struct Mol Biol, Vol. 19, Issue 9, Page 893-899, Year 2012 |
| 掲載日 | 2012年8月5日 |
 著者 著者 | Youdong Mao / Liping Wang / Christopher Gu / Alon Herschhorn / Shi-Hua Xiang / Hillel Haim / Xinzhen Yang / Joseph Sodroski /  |
| PubMed 要旨 | The trimeric human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein (Env) spike is a molecular machine that mediates virus entry into host cells and is the sole target for virus- ...The trimeric human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein (Env) spike is a molecular machine that mediates virus entry into host cells and is the sole target for virus-neutralizing antibodies. The mature Env spike results from cleavage of a trimeric glycoprotein precursor, gp160, into three gp120 and three gp41 subunits. Here, we describe an ~11-Å cryo-EM structure of the trimeric HIV-1 Env precursor in its unliganded state. The three gp120 and three gp41 subunits form a cage-like structure with an interior void surrounding the trimer axis. Interprotomer contacts are limited to the gp41 transmembrane region, the torus-like gp41 ectodomain and a trimer-association domain of gp120 composed of the V1, V2 and V3 variable regions. The cage-like architecture, which is unique among characterized viral envelope proteins, restricts antibody access, reflecting requirements imposed by HIV-1 persistence in the host. |
 リンク リンク |  Nat Struct Mol Biol / Nat Struct Mol Biol /  PubMed:22864288 / PubMed:22864288 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) |
| 解像度 | 10.8 Å |
| 構造データ | 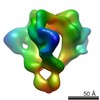 EMDB-5418: |
| 由来 |
|
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について




 Human immunodeficiency virus 1 (ヒト免疫不全ウイルス)
Human immunodeficiency virus 1 (ヒト免疫不全ウイルス)