+検索条件
-Structure paper
| タイトル | Cryo-EM structure of the β3-adrenergic receptor reveals the molecular basis of subtype selectivity. |
|---|---|
| ジャーナル・号・ページ | Mol Cell, Vol. 81, Issue 15, Page 3205-33215.e5, Year 2021 |
| 掲載日 | 2021年8月5日 |
 著者 著者 | Chisae Nagiri / Kazuhiro Kobayashi / Atsuhiro Tomita / Masahiko Kato / Kan Kobayashi / Keitaro Yamashita / Tomohiro Nishizawa / Asuka Inoue / Wataru Shihoya / Osamu Nureki /  |
| PubMed 要旨 | The β-adrenergic receptor (βAR) is predominantly expressed in adipose tissue and urinary bladder and has emerged as an attractive drug target for the treatment of type 2 diabetes, obesity, and ...The β-adrenergic receptor (βAR) is predominantly expressed in adipose tissue and urinary bladder and has emerged as an attractive drug target for the treatment of type 2 diabetes, obesity, and overactive bladder (OAB). Here, we report the cryogenic electron microscopy structure of the βAR-G signaling complex with the selective agonist mirabegron, a first-in-class drug for OAB. Comparison of this structure with the previously reported βAR and βAR structures reveals a receptor activation mechanism upon mirabegron binding to the orthosteric site. Notably, the narrower exosite in βAR creates a perpendicular pocket for mirabegron. Mutational analyses suggest that a combination of both the exosite shape and the amino-acid-residue substitutions defines the drug selectivity of the βAR agonists. Our findings provide a molecular basis for βAR subtype selectivity, allowing the design of more-selective agents with fewer adverse effects. |
 リンク リンク |  Mol Cell / Mol Cell /  PubMed:34314699 PubMed:34314699 |
| 手法 | EM (単粒子) |
| 解像度 | 3.16 - 3.3 Å |
| 構造データ | EMDB-30678, PDB-7dh5: EMDB-33227, PDB-7xjh: |
| 化合物 | 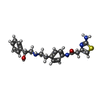 ChemComp-H6U: 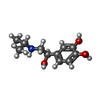 ChemComp-5FW: |
| 由来 |
|
 キーワード キーワード | MEMBRANE PROTEIN / GPCR |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について







 homo sapiens (ヒト)
homo sapiens (ヒト)
