+Search query
-Structure paper
| Title | Cryo-EM structure of the β3-adrenergic receptor reveals the molecular basis of subtype selectivity. |
|---|---|
| Journal, issue, pages | Mol Cell, Vol. 81, Issue 15, Page 3205-33215.e5, Year 2021 |
| Publish date | Aug 5, 2021 |
 Authors Authors | Chisae Nagiri / Kazuhiro Kobayashi / Atsuhiro Tomita / Masahiko Kato / Kan Kobayashi / Keitaro Yamashita / Tomohiro Nishizawa / Asuka Inoue / Wataru Shihoya / Osamu Nureki /  |
| PubMed Abstract | The β-adrenergic receptor (βAR) is predominantly expressed in adipose tissue and urinary bladder and has emerged as an attractive drug target for the treatment of type 2 diabetes, obesity, and ...The β-adrenergic receptor (βAR) is predominantly expressed in adipose tissue and urinary bladder and has emerged as an attractive drug target for the treatment of type 2 diabetes, obesity, and overactive bladder (OAB). Here, we report the cryogenic electron microscopy structure of the βAR-G signaling complex with the selective agonist mirabegron, a first-in-class drug for OAB. Comparison of this structure with the previously reported βAR and βAR structures reveals a receptor activation mechanism upon mirabegron binding to the orthosteric site. Notably, the narrower exosite in βAR creates a perpendicular pocket for mirabegron. Mutational analyses suggest that a combination of both the exosite shape and the amino-acid-residue substitutions defines the drug selectivity of the βAR agonists. Our findings provide a molecular basis for βAR subtype selectivity, allowing the design of more-selective agents with fewer adverse effects. |
 External links External links |  Mol Cell / Mol Cell /  PubMed:34314699 PubMed:34314699 |
| Methods | EM (single particle) |
| Resolution | 3.16 - 3.3 Å |
| Structure data | EMDB-30678, PDB-7dh5: EMDB-33227, PDB-7xjh: |
| Chemicals | 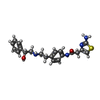 ChemComp-H6U: 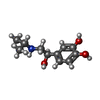 ChemComp-5FW: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN / GPCR |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers







 homo sapiens (human)
homo sapiens (human)
