+検索条件
-Structure paper
| タイトル | Molecular basis for ligand activation of the human KCNQ2 channel. |
|---|---|
| ジャーナル・号・ページ | Cell Res, Vol. 31, Issue 1, Page 52-61, Year 2021 |
| 掲載日 | 2020年9月3日 |
 著者 著者 | Xiaoxiao Li / Qiansen Zhang / Peipei Guo / Jie Fu / Lianghe Mei / Dashuai Lv / Jiangqin Wang / Dongwu Lai / Sheng Ye / Huaiyu Yang / Jiangtao Guo /  |
| PubMed 要旨 | The voltage-gated potassium channel KCNQ2 is responsible for M-current in neurons and is an important drug target to treat epilepsy, pain and several other diseases related to neuronal hyper- ...The voltage-gated potassium channel KCNQ2 is responsible for M-current in neurons and is an important drug target to treat epilepsy, pain and several other diseases related to neuronal hyper-excitability. A list of synthetic compounds have been developed to directly activate KCNQ2, yet our knowledge of their activation mechanism is limited, due to lack of high-resolution structures. Here, we report cryo-electron microscopy (cryo-EM) structures of the human KCNQ2 determined in apo state and in complex with two activators, ztz240 or retigabine, which activate KCNQ2 through different mechanisms. The activator-bound structures, along with electrophysiology analysis, reveal that ztz240 binds at the voltage-sensing domain and directly stabilizes it at the activated state, whereas retigabine binds at the pore domain and activates the channel by an allosteric modulation. By accurately defining ligand-binding sites, these KCNQ2 structures not only reveal different ligand recognition and activation mechanisms, but also provide a structural basis for drug optimization and design. |
 リンク リンク |  Cell Res / Cell Res /  PubMed:32884139 / PubMed:32884139 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) |
| 解像度 | 3.1 - 3.9 Å |
| 構造データ | EMDB-30443, PDB-7cr0: EMDB-30444, PDB-7cr1: EMDB-30445, PDB-7cr2: EMDB-30446, PDB-7cr3: EMDB-30447, PDB-7cr4: EMDB-30448, PDB-7cr7: |
| 化合物 | 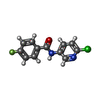 ChemComp-GB9: 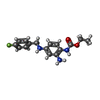 ChemComp-FBX: |
| 由来 |
|
 キーワード キーワード | TRANSPORT PROTEIN / ion channel |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について















 homo sapiens (ヒト)
homo sapiens (ヒト)