+Search query
-Structure paper
| Title | Structural basis of the substrate recognition and inhibition mechanism of Plasmodium falciparum nucleoside transporter PfENT1. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 14, Issue 1, Page 1727, Year 2023 |
| Publish date | Mar 28, 2023 |
 Authors Authors | Chen Wang / Leiye Yu / Jiying Zhang / Yanxia Zhou / Bo Sun / Qingjie Xiao / Minhua Zhang / Huayi Liu / Jinhong Li / Jialu Li / Yunzi Luo / Jie Xu / Zhong Lian / Jingwen Lin / Xiang Wang / Peng Zhang / Li Guo / Ruobing Ren / Dong Deng /  |
| PubMed Abstract | By lacking de novo purine biosynthesis enzymes, Plasmodium falciparum requires purine nucleoside uptake from host cells. The indispensable nucleoside transporter ENT1 of P. falciparum facilitates ...By lacking de novo purine biosynthesis enzymes, Plasmodium falciparum requires purine nucleoside uptake from host cells. The indispensable nucleoside transporter ENT1 of P. falciparum facilitates nucleoside uptake in the asexual blood stage. Specific inhibitors of PfENT1 prevent the proliferation of P. falciparum at submicromolar concentrations. However, the substrate recognition and inhibitory mechanism of PfENT1 are still elusive. Here, we report cryo-EM structures of PfENT1 in apo, inosine-bound, and inhibitor-bound states. Together with in vitro binding and uptake assays, we identify that inosine is the primary substrate of PfENT1 and that the inosine-binding site is located in the central cavity of PfENT1. The endofacial inhibitor GSK4 occupies the orthosteric site of PfENT1 and explores the allosteric site to block the conformational change of PfENT1. Furthermore, we propose a general "rocker switch" alternating access cycle for ENT transporters. Understanding the substrate recognition and inhibitory mechanisms of PfENT1 will greatly facilitate future efforts in the rational design of antimalarial drugs. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:36977719 / PubMed:36977719 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 3.11 - 4.04 Å |
| Structure data | EMDB-32618, PDB-7wn0: EMDB-32619, PDB-7wn1: EMDB-33756, PDB-7ydq: |
| Chemicals | 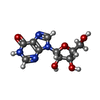 ChemComp-NOS: 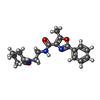 ChemComp-IRX: |
| Source |
|
 Keywords Keywords | TRANSPORT PROTEIN / malaria / nucleoside transporter / Equilibrative nucleoside transporter / inosine / MEMBRANE PROTEIN / GSK4 |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers












