+Search query
-Structure paper
| Title | Structural and mechanistic insights into fungal β-1,3-glucan synthase FKS1. |
|---|---|
| Journal, issue, pages | Nature, Vol. 616, Issue 7955, Page 190-198, Year 2023 |
| Publish date | Mar 22, 2023 |
 Authors Authors | Xinlin Hu / Ping Yang / Changdong Chai / Jia Liu / Huanhuan Sun / Yanan Wu / Mingjie Zhang / Min Zhang / Xiaotian Liu / Hongjun Yu /  |
| PubMed Abstract | The membrane-integrated synthase FKS is involved in the biosynthesis of β-1,3-glucan, the core component of the fungal cell wall. FKS is the target of widely prescribed antifungal drugs, including ...The membrane-integrated synthase FKS is involved in the biosynthesis of β-1,3-glucan, the core component of the fungal cell wall. FKS is the target of widely prescribed antifungal drugs, including echinocandin and ibrexafungerp. Unfortunately, the mechanism of action of FKS remains enigmatic and this has hampered development of more effective medicines targeting the enzyme. Here we present the cryo-electron microscopy structures of Saccharomyces cerevisiae FKS1 and the echinocandin-resistant mutant FKS1(S643P). These structures reveal the active site of the enzyme at the membrane-cytoplasm interface and a glucan translocation path spanning the membrane bilayer. Multiple bound lipids and notable membrane distortions are observed in the FKS1 structures, suggesting active FKS1-membrane interactions. Echinocandin-resistant mutations are clustered at a region near TM5-6 and TM8 of FKS1. The structure of FKS1(S643P) reveals altered lipid arrangements in this region, suggesting a drug-resistant mechanism of the mutant enzyme. The structures, the catalytic mechanism and the molecular insights into drug-resistant mutations of FKS1 revealed in this study advance the mechanistic understanding of fungal β-1,3-glucan biosynthesis and establish a foundation for developing new antifungal drugs by targeting FKS. |
 External links External links |  Nature / Nature /  PubMed:36949198 / PubMed:36949198 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 3.4 - 3.5 Å |
| Structure data | EMDB-33154, PDB-7xe4: EMDB-34115, PDB-7yuy: |
| Chemicals | 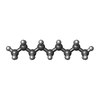 ChemComp-DD9:  ChemComp-D10:  ChemComp-C14: 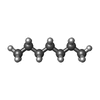 ChemComp-HP6: 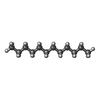 ChemComp-D12: 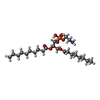 ChemComp-XKP: |
| Source |
|
 Keywords Keywords | TRANSFERASE / membrane protein / glycosyltransferase |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers








