+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7yuy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of a mutated membrane-bound glycosyltransferase | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Components Components | 1,3-beta-glucan synthase component FKS1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | TRANSFERASE / membrane protein / glycosyltransferase | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationfungal-type cell wall polysaccharide biosynthetic process / 1,3-beta-glucan synthase / 1,3-beta-D-glucan synthase activity / (1->3)-beta-D-glucan biosynthetic process / 1,3-beta-D-glucan synthase complex / fungal-type cell wall biogenesis / cellular bud / ascospore wall assembly / actin cortical patch / cellular bud tip ...fungal-type cell wall polysaccharide biosynthetic process / 1,3-beta-glucan synthase / 1,3-beta-D-glucan synthase activity / (1->3)-beta-D-glucan biosynthetic process / 1,3-beta-D-glucan synthase complex / fungal-type cell wall biogenesis / cellular bud / ascospore wall assembly / actin cortical patch / cellular bud tip / fungal-type cell wall / cellular bud neck / regulation of cell size / positive regulation of endocytosis / cell periphery / mitochondrion / plasma membrane Similarity search - Function | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological species |  | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Hu, X.L. / Yang, P. / Zhang, M. / Liu, X.T. / Yu, H.J. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Funding support |  China, 1items China, 1items
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Structural and mechanistic insights into fungal β-1,3-glucan synthase FKS1. Authors: Xinlin Hu / Ping Yang / Changdong Chai / Jia Liu / Huanhuan Sun / Yanan Wu / Mingjie Zhang / Min Zhang / Xiaotian Liu / Hongjun Yu /  Abstract: The membrane-integrated synthase FKS is involved in the biosynthesis of β-1,3-glucan, the core component of the fungal cell wall. FKS is the target of widely prescribed antifungal drugs, including ...The membrane-integrated synthase FKS is involved in the biosynthesis of β-1,3-glucan, the core component of the fungal cell wall. FKS is the target of widely prescribed antifungal drugs, including echinocandin and ibrexafungerp. Unfortunately, the mechanism of action of FKS remains enigmatic and this has hampered development of more effective medicines targeting the enzyme. Here we present the cryo-electron microscopy structures of Saccharomyces cerevisiae FKS1 and the echinocandin-resistant mutant FKS1(S643P). These structures reveal the active site of the enzyme at the membrane-cytoplasm interface and a glucan translocation path spanning the membrane bilayer. Multiple bound lipids and notable membrane distortions are observed in the FKS1 structures, suggesting active FKS1-membrane interactions. Echinocandin-resistant mutations are clustered at a region near TM5-6 and TM8 of FKS1. The structure of FKS1(S643P) reveals altered lipid arrangements in this region, suggesting a drug-resistant mechanism of the mutant enzyme. The structures, the catalytic mechanism and the molecular insights into drug-resistant mutations of FKS1 revealed in this study advance the mechanistic understanding of fungal β-1,3-glucan biosynthesis and establish a foundation for developing new antifungal drugs by targeting FKS. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7yuy.cif.gz 7yuy.cif.gz | 299.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7yuy.ent.gz pdb7yuy.ent.gz | 230.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7yuy.json.gz 7yuy.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7yuy_validation.pdf.gz 7yuy_validation.pdf.gz | 1.5 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7yuy_full_validation.pdf.gz 7yuy_full_validation.pdf.gz | 1.5 MB | Display | |
| Data in XML |  7yuy_validation.xml.gz 7yuy_validation.xml.gz | 53.1 KB | Display | |
| Data in CIF |  7yuy_validation.cif.gz 7yuy_validation.cif.gz | 82.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yu/7yuy https://data.pdbj.org/pub/pdb/validation_reports/yu/7yuy ftp://data.pdbj.org/pub/pdb/validation_reports/yu/7yuy ftp://data.pdbj.org/pub/pdb/validation_reports/yu/7yuy | HTTPS FTP |
-Related structure data
| Related structure data |  34115MC  7xe4C C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 1 types, 1 molecules F
| #1: Protein | Mass: 215086.203 Da / Num. of mol.: 1 / Mutation: S643P Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Strain: ATCC 204508 / S288c Gene: FKS1, CND1, CWH53, ETG1, GLS1, GSC1, PBR1, YLR342W, L8300.6 Production host:  |
|---|
-Sugars , 2 types, 2 molecules
| #2: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source |
|---|---|
| #3: Polysaccharide | beta-D-glucopyranose-(1-3)-beta-D-glucopyranose-(1-3)-beta-D-glucopyranose-(1-3)-beta-D-glucopyranose |
-Non-polymers , 6 types, 25 molecules 




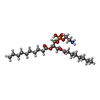





| #4: Chemical | ChemComp-DD9 / #5: Chemical | ChemComp-D10 / #6: Chemical | ChemComp-C14 / | #7: Chemical | ChemComp-HP6 / #8: Chemical | #9: Chemical | ChemComp-XKP / ( | |
|---|
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: membrane-bound glycosyltransferase / Type: COMPLEX / Entity ID: #1 / Source: NATURAL |
|---|---|
| Molecular weight | Value: 0.215 MDa / Experimental value: NO |
| Source (natural) | Organism:  |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 3000 nm / Nominal defocus min: 1100 nm |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.19.1_4122: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 176682 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller




 PDBj
PDBj





