+Search query
-Structure paper
| Title | Identification of a conserved virion-stabilizing network inside the interprotomer pocket of enteroviruses. |
|---|---|
| Journal, issue, pages | Commun Biol, Vol. 4, Issue 1, Page 250, Year 2021 |
| Publish date | Feb 26, 2021 |
 Authors Authors | Justin W Flatt / Aušra Domanska / Alma L Seppälä / Sarah J Butcher /  |
| PubMed Abstract | Enteroviruses pose a persistent and widespread threat to human physical health, with no specific treatments available. Small molecule capsid binders have the potential to be developed as antivirals ...Enteroviruses pose a persistent and widespread threat to human physical health, with no specific treatments available. Small molecule capsid binders have the potential to be developed as antivirals that prevent virus attachment and entry into host cells. To aid with broad-range drug development, we report here structures of coxsackieviruses B3 and B4 bound to different interprotomer-targeting capsid binders using single-particle cryo-EM. The EM density maps are beyond 3 Å resolution, providing detailed information about interactions in the ligand-binding pocket. Comparative analysis revealed the residues that form a conserved virion-stabilizing network at the interprotomer site, and showed the small molecule properties that allow anchoring in the pocket to inhibit virus disassembly. |
 External links External links |  Commun Biol / Commun Biol /  PubMed:33637854 / PubMed:33637854 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.7 - 3.4 Å |
| Structure data | EMDB-11165, PDB-6zck: EMDB-11166, PDB-6zcl: EMDB-11300, PDB-6zms: |
| Chemicals | 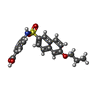 ChemComp-QFW: 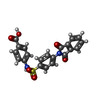 ChemComp-FHK:  ChemComp-MYR: |
| Source |
|
 Keywords Keywords | VIRUS / Enterovirus / Coxsackievirus B4 / Inhibitor / Capsid Binder / coxackievirus B4 |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers









 coxsackievirus b4 (strain e2)
coxsackievirus b4 (strain e2)