+検索条件
-Structure paper
| タイトル | Cryo-EM structures of apo and atorvastatin-bound human 3-hydroxy-3-methylglutaryl-coenzyme A reductase. |
|---|---|
| ジャーナル・号・ページ | Acta Crystallogr F Struct Biol Commun, Vol. 81, Issue Pt 3, Page 118-122, Year 2025 |
| 掲載日 | 2025年3月1日 |
 著者 著者 | Manikandan Karuppasamy / Jason van Rooyen /  |
| PubMed 要旨 | The enzyme 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR) regulates the level of cholesterol by catalysing the formation/production of mevalonate and has therefore become an important ...The enzyme 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR) regulates the level of cholesterol by catalysing the formation/production of mevalonate and has therefore become an important pharmaceutical target for coronary heart disease. Here, we report the cryo-EM structure of the catalytic part of the enzyme in the apo form and bound with its inhibitor atorvastatin, a commonly used drug in cardiovascular disease, at resolutions of 2.1 and 2.3 Å, respectively. In the cryo-EM maps, part of the N-domain corresponding to amino acids 439-487 is well ordered and could be modelled completely. Atorvastatin molecules were found to occupy all four active sites of the tetrameric complex, and the binding does not alter the conformation of the protein or the active site. The method described here exploits graphene oxide as an additional support and could be used as an alternative to elucidate the structures of pharmaceutical target compounds that are difficult to co-crystallize with human HMGR and for sparsely available samples in drug discovery. |
 リンク リンク |  Acta Crystallogr F Struct Biol Commun / Acta Crystallogr F Struct Biol Commun /  PubMed:39976191 / PubMed:39976191 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) |
| 解像度 | 2.06 - 2.26 Å |
| 構造データ | EMDB-17748, PDB-8pkn: EMDB-19757, PDB-8s6b: |
| 化合物 | 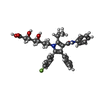 ChemComp-117: |
| 由来 |
|
 キーワード キーワード | OXIDOREDUCTASE / reductase / cholesterol biosynthesis / lipitor / atorvastatin / statins |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について







 homo sapiens (ヒト)
homo sapiens (ヒト)