+Search query
-Structure paper
| Title | Structural insights into TRAP association with ribosome-Sec61 complex and translocon inhibition by a CADA derivative. |
|---|---|
| Journal, issue, pages | Sci Adv, Vol. 9, Issue 9, Page eadf0797, Year 2023 |
| Publish date | Mar 3, 2023 |
 Authors Authors | Eva Pauwels / Neesha R Shewakramani / Brent De Wijngaert / Anita Camps / Becky Provinciael / Joren Stroobants / Kai-Uwe Kalies / Enno Hartmann / Piet Maes / Kurt Vermeire / Kalyan Das /   |
| PubMed Abstract | During cotranslational translocation, the signal peptide of a nascent chain binds Sec61 translocon to initiate protein transport through the endoplasmic reticulum (ER) membrane. Our cryo-electron ...During cotranslational translocation, the signal peptide of a nascent chain binds Sec61 translocon to initiate protein transport through the endoplasmic reticulum (ER) membrane. Our cryo-electron microscopy structure of ribosome-Sec61 shows binding of an ordered heterotetrameric translocon-associated protein (TRAP) complex, in which TRAP-γ is anchored at two adjacent positions of 28 ribosomal RNA and interacts with ribosomal protein L38 and Sec61α/γ. Four transmembrane helices (TMHs) of TRAP-γ cluster with one C-terminal helix of each α, β, and δ subunits. The seven TMH bundle helps position a crescent-shaped trimeric TRAP-α/β/δ core in the ER lumen, facing the Sec61 channel. Further, our in vitro assay establishes the cyclotriazadisulfonamide derivative CK147 as a translocon inhibitor. A structure of ribosome-Sec61-CK147 reveals CK147 binding the channel and interacting with the plug helix from the lumenal side. The CK147 resistance mutations surround the inhibitor. These structures help in understanding the TRAP functions and provide a new Sec61 site for designing translocon inhibitors. |
 External links External links |  Sci Adv / Sci Adv /  PubMed:36867692 / PubMed:36867692 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.79 - 2.86 Å |
| Structure data | EMDB-15860, PDB-8b5l: EMDB-15863, PDB-8b6c: |
| Chemicals |  ChemComp-MG:  ChemComp-ZN: 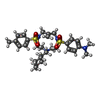 ChemComp-OWI: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN / Ribosome / Sec61 / TRAP / Translocon / Translation / RNA binding protein / eEF2 / CK147 / CADA / Translocon inhibitor / 80S / 60S / 40S / cyclotriazadisulfonamide |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers








