+Search query
-Structure paper
| Title | The antigenic anatomy of SARS-CoV-2 receptor binding domain. |
|---|---|
| Journal, issue, pages | Cell, Vol. 184, Issue 8, Page 2183-2200.e22, Year 2021 |
| Publish date | Apr 15, 2021 |
 Authors Authors | Wanwisa Dejnirattisai / Daming Zhou / Helen M Ginn / Helen M E Duyvesteyn / Piyada Supasa / James Brett Case / Yuguang Zhao / Thomas S Walter / Alexander J Mentzer / Chang Liu / Beibei Wang / Guido C Paesen / Jose Slon-Campos / César López-Camacho / Natasha M Kafai / Adam L Bailey / Rita E Chen / Baoling Ying / Craig Thompson / Jai Bolton / Alex Fyfe / Sunetra Gupta / Tiong Kit Tan / Javier Gilbert-Jaramillo / William James / Michael Knight / Miles W Carroll / Donal Skelly / Christina Dold / Yanchun Peng / Robert Levin / Tao Dong / Andrew J Pollard / Julian C Knight / Paul Klenerman / Nigel Temperton / David R Hall / Mark A Williams / Neil G Paterson / Felicity K R Bertram / C Alistair Siebert / Daniel K Clare / Andrew Howe / Julika Radecke / Yun Song / Alain R Townsend / Kuan-Ying A Huang / Elizabeth E Fry / Juthathip Mongkolsapaya / Michael S Diamond / Jingshan Ren / David I Stuart / Gavin R Screaton /     |
| PubMed Abstract | Antibodies are crucial to immune protection against SARS-CoV-2, with some in emergency use as therapeutics. Here, we identify 377 human monoclonal antibodies (mAbs) recognizing the virus spike and ...Antibodies are crucial to immune protection against SARS-CoV-2, with some in emergency use as therapeutics. Here, we identify 377 human monoclonal antibodies (mAbs) recognizing the virus spike and focus mainly on 80 that bind the receptor binding domain (RBD). We devise a competition data-driven method to map RBD binding sites. We find that although antibody binding sites are widely dispersed, neutralizing antibody binding is focused, with nearly all highly inhibitory mAbs (IC < 0.1 μg/mL) blocking receptor interaction, except for one that binds a unique epitope in the N-terminal domain. Many of these neutralizing mAbs use public V-genes and are close to germline. We dissect the structural basis of recognition for this large panel of antibodies through X-ray crystallography and cryoelectron microscopy of 19 Fab-antigen structures. We find novel binding modes for some potently inhibitory antibodies and demonstrate that strongly neutralizing mAbs protect, prophylactically or therapeutically, in animal models. |
 External links External links |  Cell / Cell /  PubMed:33756110 / PubMed:33756110 /  PubMed Central PubMed Central |
| Methods | EM (single particle) / X-ray diffraction |
| Resolution | 2.04 - 7.3 Å |
| Structure data | EMDB-12274, PDB-7nd3: EMDB-12275, PDB-7nd4: EMDB-12276, PDB-7nd5: EMDB-12277, PDB-7nd6: EMDB-12278, PDB-7nd7: EMDB-12279, PDB-7nd8: EMDB-12280, PDB-7nd9: EMDB-12281, PDB-7nda: EMDB-12282, PDB-7ndb: EMDB-12283, PDB-7ndc: EMDB-12284, PDB-7ndd:  PDB-7beh:  PDB-7bei:  PDB-7bej:  PDB-7bek:  PDB-7bel:  PDB-7bem:  PDB-7ben:  PDB-7beo:  PDB-7bep: |
| Chemicals |  ChemComp-GOL:  ChemComp-TRS:  ChemComp-HOH:  ChemComp-NO3:  ChemComp-CL:  ChemComp-FMT:  ChemComp-PEG:  ChemComp-SO4:  ChemComp-ACT:  ChemComp-NAG:  ChemComp-PO4: 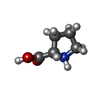 ChemComp-PRO:  ChemComp-IMD:  ChemComp-BR: 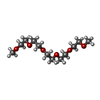 ChemComp-PG6:  ChemComp-IOD:  ChemComp-GLU:  ChemComp-GLY:  ChemComp-PGE:  ChemComp-PG4: |
| Source |
|
 Keywords Keywords | VIRAL PROTEIN/IMMUNE SYSTEM / SARS-CoV-2 / antibody / germline / V-gene / receptor-binding-domain / spike / neutralisation / protection / glycosylation / valency / VIRAL PROTEIN / IMMUNE SYSTEM / VIRAL PROTEIN-IMMUNE SYSTEM complex |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers


























 homo sapiens (human)
homo sapiens (human)