+検索条件
-Structure paper
| タイトル | The Unusual Homodimer of a Heme-Copper Terminal Oxidase Allows Itself to Utilize Two Electron Donors. |
|---|---|
| ジャーナル・号・ページ | Angew Chem Int Ed Engl, Vol. 60, Issue 24, Page 13323-13330, Year 2021 |
| 掲載日 | 2021年6月7日 |
 著者 著者 | Guoliang Zhu / Hui Zeng / Shuangbo Zhang / Jana Juli / Linhua Tai / Danyang Zhang / Xiaoyun Pang / Yan Zhang / Sin Man Lam / Yun Zhu / Guohong Peng / Hartmut Michel / Fei Sun /   |
| PubMed 要旨 | The heme-copper oxidase superfamily comprises cytochrome c and ubiquinol oxidases. These enzymes catalyze the transfer of electrons from different electron donors onto molecular oxygen. A B-family ...The heme-copper oxidase superfamily comprises cytochrome c and ubiquinol oxidases. These enzymes catalyze the transfer of electrons from different electron donors onto molecular oxygen. A B-family cytochrome c oxidase from the hyperthermophilic bacterium Aquifex aeolicus was discovered previously to be able to use both cytochrome c and naphthoquinol as electron donors. Its molecular mechanism as well as the evolutionary significance are yet unknown. Here we solved its 3.4 Å resolution electron cryo-microscopic structure and discovered a novel dimeric structure mediated by subunit I (CoxA2) that would be essential for naphthoquinol binding and oxidation. The unique structural features in both proton and oxygen pathways suggest an evolutionary adaptation of this oxidase to its hyperthermophilic environment. Our results add a new conceptual understanding of structural variation of cytochrome c oxidases in different species. |
 リンク リンク |  Angew Chem Int Ed Engl / Angew Chem Int Ed Engl /  PubMed:33665933 / PubMed:33665933 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) |
| 解像度 | 3.4 Å |
| 構造データ | EMDB-30657, PDB-7deg: |
| 化合物 | 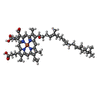 ChemComp-HAS:  ChemComp-HEM:  ChemComp-CU: 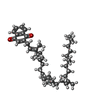 ChemComp-DLX:  ChemComp-PGV:  ChemComp-3PE:  ChemComp-CUA: |
| 由来 |
|
 キーワード キーワード | OXIDOREDUCTASE / electron cryo-microscopy / heme-copper oxidase / cytochrome c oxidase dimer / Aquifex aeolicus / naphthoquinone |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について






 Aquifex aeolicus VF5 (バクテリア)
Aquifex aeolicus VF5 (バクテリア)