+Search query
-Structure paper
| Title | The internal interaction in RBBP5 regulates assembly and activity of MLL1 methyltransferase complex. |
|---|---|
| Journal, issue, pages | Nucleic Acids Res, Vol. 47, Issue 19, Page 10426-10438, Year 2019 |
| Publish date | Nov 4, 2019 |
 Authors Authors | Jianming Han / Tingting Li / Yanjing Li / Muchun Li / Xiaoman Wang / Chao Peng / Chen Su / Na Li / Yiwen Li / Ying Xu / Yong Chen /  |
| PubMed Abstract | The Mixed Lineage Leukemia protein 1 (MLL1) plays an essential role in the maintenance of the histone H3 lysine 4 (H3K4) methylation status for gene expression during differentiation and development. ...The Mixed Lineage Leukemia protein 1 (MLL1) plays an essential role in the maintenance of the histone H3 lysine 4 (H3K4) methylation status for gene expression during differentiation and development. The methyltransferase activity of MLL1 is regulated by three conserved core subunits, WDR5, RBBP5 and ASH2L. Here, we determined the structure of human RBBP5 and demonstrated its role in the assembly and regulation of the MLL1 complex. We identified an internal interaction between the WD40 propeller and the C-terminal distal region in RBBP5, which assisted the maintenance of the compact conformation of the MLL1 complex. We also discovered a vertebrate-specific motif in the C-terminal distal region of RBBP5 that contributed to nucleosome recognition and methylation of nucleosomes by the MLL1 complex. Our results provide new insights into functional conservation and evolutionary plasticity of the scaffold protein RBBP5 in the regulation of KMT2-family methyltransferase complexes. |
 External links External links |  Nucleic Acids Res / Nucleic Acids Res /  PubMed:31544921 / PubMed:31544921 /  PubMed Central PubMed Central |
| Methods | SAS (X-ray synchrotron) / X-ray diffraction |
| Resolution | 1.801 Å |
| Structure data | 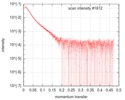 SASDGD4: Mixed lineage leukemia protein-1 complex, MLL1-WDR5-ASH2L-RBBP5(2-381)  SASDGE4: Mixed lineage leukemia protein-1 complex, MLL1-WDR5-ASH2L-RBBP5(2-480) 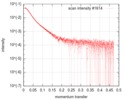 SASDGF4: Mixed lineage leukemia protein-1 complex, MLL1-WDR5-ASH2L-RBBP5(2-480)L399A/L400A/I457A/L459A 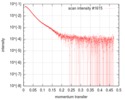 SASDGG4: Mixed lineage leukemia protein-1 complex, MLL1-WDR5-ASH2L-RBBP5(2-538)  PDB-6km7: |
| Chemicals |  ChemComp-SO4:  ChemComp-2PE:  ChemComp-P6G:  ChemComp-PG4:  ChemComp-HOH: |
| Source |
|
 Keywords Keywords | PROTEIN BINDING / Histone methyltransferase / MLL1 complex / RBBP5 / histone methylation / epigenetics |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers



 homo sapiens (human)
homo sapiens (human)