+Search query
-Structure paper
| Title | Conformational transitions of the serotonin 5-HT receptor. |
|---|---|
| Journal, issue, pages | Nature, Vol. 563, Issue 7730, Page 275-279, Year 2018 |
| Publish date | Oct 31, 2018 |
 Authors Authors | Lucie Polovinkin / Ghérici Hassaine / Jonathan Perot / Emmanuelle Neumann / Anders A Jensen / Solène N Lefebvre / Pierre-Jean Corringer / Jacques Neyton / Christophe Chipot / Francois Dehez / Guy Schoehn / Hugues Nury /    |
| PubMed Abstract | The serotonin 5-HT receptor is a pentameric ligand-gated ion channel (pLGIC). It belongs to a large family of receptors that function as allosteric signal transducers across the plasma membrane; upon ...The serotonin 5-HT receptor is a pentameric ligand-gated ion channel (pLGIC). It belongs to a large family of receptors that function as allosteric signal transducers across the plasma membrane; upon binding of neurotransmitter molecules to extracellular sites, the receptors undergo complex conformational transitions that result in transient opening of a pore permeable to ions. 5-HT receptors are therapeutic targets for emesis and nausea, irritable bowel syndrome and depression. In spite of several reported pLGIC structures, no clear unifying view has emerged on the conformational transitions involved in channel gating. Here we report four cryo-electron microscopy structures of the full-length mouse 5-HT receptor in complex with the anti-emetic drug tropisetron, with serotonin, and with serotonin and a positive allosteric modulator, at resolutions ranging from 3.2 Å to 4.5 Å. The tropisetron-bound structure resembles those obtained with an inhibitory nanobody or without ligand. The other structures include an 'open' state and two ligand-bound states. We present computational insights into the dynamics of the structures, their pore hydration and free-energy profiles, and characterize movements at the gate level and cation accessibility in the pore. Together, these data deepen our understanding of the gating mechanism of pLGICs and capture ligand binding in unprecedented detail. |
 External links External links |  Nature / Nature /  PubMed:30401839 / PubMed:30401839 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 3.2 - 4.5 Å |
| Structure data | EMDB-0225, PDB-6hin: EMDB-0226, PDB-6hio: |
| Chemicals |  ChemComp-NAG: 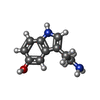 ChemComp-SRO: 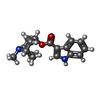 ChemComp-TKT: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN / Ion channel / serotonin receptor / pentameric ligand-gated channel |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers












