+検索条件
-Structure paper
| タイトル | Cryo-EM structure of the Blastochloris viridis LH1-RC complex at 2.9 Å. |
|---|---|
| ジャーナル・号・ページ | Nature, Vol. 556, Issue 7700, Page 203-208, Year 2018 |
| 掲載日 | 2018年4月4日 |
 著者 著者 | Pu Qian / C Alistair Siebert / Peiyi Wang / Daniel P Canniffe / C Neil Hunter /  |
| PubMed 要旨 | The light-harvesting 1-reaction centre (LH1-RC) complex is a key functional component of bacterial photosynthesis. Here we present a 2.9 Å resolution cryo-electron microscopy structure of the ...The light-harvesting 1-reaction centre (LH1-RC) complex is a key functional component of bacterial photosynthesis. Here we present a 2.9 Å resolution cryo-electron microscopy structure of the bacteriochlorophyll b-based LH1-RC complex from Blastochloris viridis that reveals the structural basis for absorption of infrared light and the molecular mechanism of quinone migration across the LH1 complex. The triple-ring LH1 complex comprises a circular array of 17 β-polypeptides sandwiched between 17 α- and 16 γ-polypeptides. Tight packing of the γ-apoproteins between β-polypeptides collectively interlocks and stabilizes the LH1 structure; this, together with the short Mg-Mg distances of bacteriochlorophyll b pairs, contributes to the large redshift of bacteriochlorophyll b absorption. The 'missing' 17th γ-polypeptide creates a pore in the LH1 ring, and an adjacent binding pocket provides a folding template for a quinone, Q , which adopts a compact, export-ready conformation before passage through the pore and eventual diffusion to the cytochrome bc complex. |
 リンク リンク |  Nature / Nature /  PubMed:29618818 PubMed:29618818 |
| 手法 | EM (単粒子) |
| 解像度 | 2.87 Å |
| 構造データ | |
| 化合物 |  ChemComp-HEM: 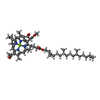 ChemComp-BCB: 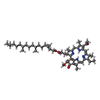 ChemComp-BPB: 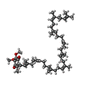 ChemComp-UQ9:  ChemComp-FE:  ChemComp-SO4: 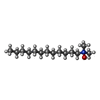 ChemComp-LDA:  ChemComp-MQ9:  ChemComp-NS5:  ChemComp-NS0: |
| 由来 |
|
 キーワード キーワード | PHOTOSYNTHESIS / Reaction centre light harvesting complex 1 Blc. viridis Cryo-EM RC-LH1 Photosynthesis |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について





 blastochloris viridis (バクテリア)
blastochloris viridis (バクテリア)