+Search query
-Structure paper
| Title | Cryo-electron Microscopy Structures of Chimeric Hemagglutinin Displayed on a Universal Influenza Vaccine Candidate. |
|---|---|
| Journal, issue, pages | mBio, Vol. 7, Issue 2, Page e00257, Year 2016 |
| Publish date | Mar 22, 2016 |
 Authors Authors | Erin E H Tran / Kira A Podolsky / Alberto Bartesaghi / Oleg Kuybeda / Giovanna Grandinetti / Teddy John Wohlbold / Gene S Tan / Raffael Nachbagauer / Peter Palese / Florian Krammer / Sriram Subramaniam /   |
| PubMed Abstract | Influenza viruses expressing chimeric hemagglutinins (HAs) are important tools in the quest for a universal vaccine. Using cryo-electron tomography, we have determined the structures of a chimeric HA ...Influenza viruses expressing chimeric hemagglutinins (HAs) are important tools in the quest for a universal vaccine. Using cryo-electron tomography, we have determined the structures of a chimeric HA variant that comprises an H1 stalk and an H5 globular head domain (cH5/1 HA) in native and antibody-bound states. We show that cH5/1 HA is structurally different from native HA, displaying a 60° rotation between the stalk and head groups, leading to a novel and unexpected "open" arrangement of HA trimers. cH5/1N1 viruses also display higher glycoprotein density than pH1N1 or H5N1 viruses, but despite these differences, antibodies that target either the stalk or head domains of hemagglutinins still bind to cH5/1 HA with the same consequences as those observed with native H1 or H5 HA. Our results show that a large range of structural plasticity can be tolerated in the chimeric spike scaffold without disrupting structural and geometric aspects of antibody binding. IMPORTANCE: Chimeric hemagglutinin proteins are set to undergo human clinical trials as a universal influenza vaccine candidate, yet no structural information for these proteins is available. Using cryo-electron tomography, we report the first three-dimensional (3D) visualization of chimeric hemagglutinin proteins displayed on the surface of the influenza virus. We show that, unexpectedly, the chimeric hemagglutinin structure differs from those of naturally occurring hemagglutinins by displaying a more open head domain and a dramatically twisted head/stalk arrangement. Despite this unusual spatial relationship between head and stalk regions, virus preparations expressing the chimeric hemagglutinin are fully infectious and display a high glycoprotein density, which likely helps induction of a broadly protective immune response. |
 External links External links |  mBio / mBio /  PubMed:27006464 / PubMed:27006464 /  PubMed Central PubMed Central |
| Methods | EM (subtomogram averaging) |
| Resolution | 25.0 Å |
| Structure data |  EMDB-6607:  EMDB-6608:  EMDB-6609:  EMDB-6610:  EMDB-6611: 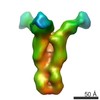 EMDB-6612: 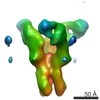 EMDB-6613:  EMDB-6614: |
| Source |
|
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers




 unidentified influenza virus
unidentified influenza virus
