+Search query
-Structure paper
| Title | Inter-ring rotations of AAA ATPase p97 revealed by electron cryomicroscopy. |
|---|---|
| Journal, issue, pages | Open Biol, Vol. 4, Issue 3, Page 130142, Year 2014 |
| Publish date | Mar 5, 2014 |
 Authors Authors | Heidi O Yeung / Andreas Förster / Cecilia Bebeacua / Hajime Niwa / Caroline Ewens / Ciarán McKeown / Xiaodong Zhang / Paul S Freemont /  |
| PubMed Abstract | The type II AAA+ protein p97 is involved in numerous cellular activities, including endoplasmic reticulum-associated degradation, transcription activation, membrane fusion and cell-cycle control. ...The type II AAA+ protein p97 is involved in numerous cellular activities, including endoplasmic reticulum-associated degradation, transcription activation, membrane fusion and cell-cycle control. These activities are at least in part regulated by the ubiquitin system, in which p97 is thought to target ubiquitylated protein substrates within macromolecular complexes and assist in their extraction or disassembly. Although ATPase activity is essential for p97 function, little is known about how ATP binding or hydrolysis is coupled with p97 conformational changes and substrate remodelling. Here, we have used single-particle electron cryomicroscopy (cryo-EM) to study the effect of nucleotides on p97 conformation. We have identified conformational heterogeneity within the cryo-EM datasets from which we have resolved two major p97 conformations. A comparison of conformations reveals inter-ring rotations upon nucleotide binding and hydrolysis that may be linked to the remodelling of target protein complexes. |
 External links External links |  Open Biol / Open Biol /  PubMed:24598262 / PubMed:24598262 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 20.0 Å |
| Structure data | 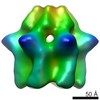 EMDB-2589:  EMDB-2590:  EMDB-2591: 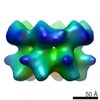 EMDB-2592: 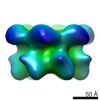 EMDB-2593: |
| Source |
|
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers




