+Search query
-Structure paper
| Title | The crystal structure of oxylipin-conjugated barley LTP1 highlights the unique plasticity of the hydrophobic cavity of these plant lipid-binding proteins. |
|---|---|
| Journal, issue, pages | Biochem. Biophys. Res. Commun., Vol. 390, Page 780-785, Year 2009 |
| Publish date | Mar 27, 2009 (structure data deposition date) |
 Authors Authors | Bakan, B. / Hamberg, M. / Larue, V. / Prange, T. / Marion, D. / Lascombe, M.B. |
 External links External links |  Biochem. Biophys. Res. Commun. / Biochem. Biophys. Res. Commun. /  PubMed:19836358 PubMed:19836358 |
| Methods | X-ray diffraction |
| Resolution | 1.8 Å |
| Structure data |  PDB-3gsh: |
| Chemicals |  ChemComp-ZN:  ChemComp-NA: 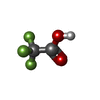 ChemComp-TFA: 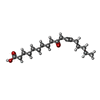 ChemComp-ASY:  ChemComp-HOH: |
| Source |
|
 Keywords Keywords | LIPID BINDING PROTEIN / LTP1 / POST-TRANSCRIPTIONAL MODIFICATION / OXYLIPIN / LIPID- BINDING / LIPOPROTEIN / TRANSPORT / Disulfide bond / Lipid-binding |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers




