+Search query
-Structure paper
| Title | Quaternary polymorphism of replicative helicase G40P: structural mapping and domain rearrangement. |
|---|---|
| Journal, issue, pages | J Mol Biol, Vol. 357, Issue 4, Page 1063-1076, Year 2006 |
| Publish date | Apr 7, 2006 |
 Authors Authors | Rafael Núñez-Ramírez / Yolanda Robledo / Pablo Mesa / Silvia Ayora / Juan Carlos Alonso / José María Carazo / Luis Enrique Donate /  |
| PubMed Abstract | Quaternary polymorphism is a distinctive structural feature of the DnaB family of replicative DNA hexameric helicases. The Bacillus subtilis bacteriophage SPP1 gene 40 product (G40P) belongs to this ...Quaternary polymorphism is a distinctive structural feature of the DnaB family of replicative DNA hexameric helicases. The Bacillus subtilis bacteriophage SPP1 gene 40 product (G40P) belongs to this family. Three different quaternary states have been described for G40P homohexamers, two of them with C(3) symmetry, and the other with C(6) symmetry. We present three-dimensional reconstructions of the different architectures of G40P hexamers and a variant lacking the N-terminal domain. Comparison of the G40P and the deletion mutant structures sheds new light on the functional roles of the N and C-terminal domains, at the same time that it allows the direct structural mapping of these domains. Based on this new information, hybrid EM/X-ray models are presented for all the different symmetries. These results suggest that quaternary polymorphism of hexameric helicases may be implicated in the translocation along the DNA. |
 External links External links |  J Mol Biol / J Mol Biol /  PubMed:16490212 PubMed:16490212 |
| Methods | EM (single particle) |
| Resolution | 22.0 - 24.0 Å |
| Structure data |  EMDB-1135:  EMDB-1146: 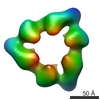 EMDB-1147: 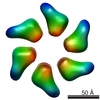 EMDB-1148: |
| Source |
|
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers



 Bacillus phage SPP1 (virus)
Bacillus phage SPP1 (virus)