+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Coxsackievirus A9 bound with compound 15 (CL278) | ||||||||||||
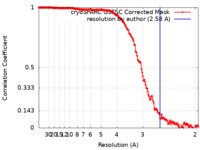
 Map data Map data | Density map of Coxsackievirus A9 bound with compound 15 (CL278) obtained by cryoEM and single-particle processing in cryoSPARC at global resolution 2.58 A. | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Antiviral / capsid stabilizer / hydrophobic pocket / cryoEM / VIRUS | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / endocytosis involved in viral entry into host cell / nucleoside-triphosphate phosphatase / channel activity ...symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / endocytosis involved in viral entry into host cell / nucleoside-triphosphate phosphatase / channel activity / monoatomic ion transmembrane transport / DNA replication / RNA helicase activity / induction by virus of host autophagy / RNA-directed RNA polymerase / viral RNA genome replication / cysteine-type endopeptidase activity / RNA-dependent RNA polymerase activity / virus-mediated perturbation of host defense response / DNA-templated transcription / host cell nucleus / virion attachment to host cell / structural molecule activity / ATP hydrolysis activity / proteolysis / RNA binding / ATP binding / membrane / metal ion binding Similarity search - Function | ||||||||||||
| Biological species |  Human coxsackievirus A9 (strain Griggs) / Human coxsackievirus A9 (strain Griggs) /  Coxsackievirus A9 Coxsackievirus A9 | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.58 Å | ||||||||||||
 Authors Authors | Plavec Z / Butcher SJ / Mitchell C / Buckner C | ||||||||||||
| Funding support |  Finland, 3 items Finland, 3 items
| ||||||||||||
 Citation Citation |  Journal: J Med Chem / Year: 2024 Journal: J Med Chem / Year: 2024Title: SAR Analysis of Novel Coxsackie virus A9 Capsid Binders. Authors: Chiara Tammaro / Zlatka Plavec / Laura Myllymäki / Cristopher Mitchell / Sara Consalvi / Mariangela Biava / Alessia Ciogli / Aušra Domanska / Valtteri Leppilampi / Cienna Buckner / Simone ...Authors: Chiara Tammaro / Zlatka Plavec / Laura Myllymäki / Cristopher Mitchell / Sara Consalvi / Mariangela Biava / Alessia Ciogli / Aušra Domanska / Valtteri Leppilampi / Cienna Buckner / Simone Manetto / Pietro Sciò / Antonio Coluccia / Mira Laajala / Giulio M Dondio / Chiara Bigogno / Varpu Marjomäki / Sarah J Butcher / Giovanna Poce /   Abstract: Enterovirus infections are common in humans, yet there are no approved antiviral treatments. In this study we concentrated on inhibition of one of the (EV-B), namely Coxsackievirus A9 (CVA9), using ...Enterovirus infections are common in humans, yet there are no approved antiviral treatments. In this study we concentrated on inhibition of one of the (EV-B), namely Coxsackievirus A9 (CVA9), using a combination of medicinal chemistry, virus inhibition assays, structure determination from cryogenic electron microscopy and molecular modeling, to determine the structure activity relationships for a promising class of novel -phenylbenzylamines. Of the new 29 compounds synthesized, 10 had half maximal effective concentration (EC) values between 0.64-10.46 μM, and of these, 7 had 50% cytotoxicity concentration (CC) values higher than 200 μM. In addition, this new series of compounds showed promising physicochemical properties and act through capsid stabilization, preventing capsid expansion and subsequent release of the genome. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_50614.map.gz emd_50614.map.gz | 172.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-50614-v30.xml emd-50614-v30.xml emd-50614.xml emd-50614.xml | 23.7 KB 23.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_50614_fsc.xml emd_50614_fsc.xml | 14.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_50614.png emd_50614.png | 69.4 KB | ||
| Filedesc metadata |  emd-50614.cif.gz emd-50614.cif.gz | 7.3 KB | ||
| Others |  emd_50614_half_map_1.map.gz emd_50614_half_map_1.map.gz emd_50614_half_map_2.map.gz emd_50614_half_map_2.map.gz | 322.6 MB 322.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-50614 http://ftp.pdbj.org/pub/emdb/structures/EMD-50614 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50614 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50614 | HTTPS FTP |
-Validation report
| Summary document |  emd_50614_validation.pdf.gz emd_50614_validation.pdf.gz | 1.3 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_50614_full_validation.pdf.gz emd_50614_full_validation.pdf.gz | 1.3 MB | Display | |
| Data in XML |  emd_50614_validation.xml.gz emd_50614_validation.xml.gz | 24.5 KB | Display | |
| Data in CIF |  emd_50614_validation.cif.gz emd_50614_validation.cif.gz | 32 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50614 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50614 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50614 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50614 | HTTPS FTP |
-Related structure data
| Related structure data |  9fo2MC  8s7jC  9exiC  9fa9C  9fczC  9fgnC  9fo5C  9fp5C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_50614.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_50614.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Density map of Coxsackievirus A9 bound with compound 15 (CL278) obtained by cryoEM and single-particle processing in cryoSPARC at global resolution 2.58 A. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.97 Å | ||||||||||||||||||||||||||||||||||||
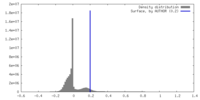
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map of Coxsackievirus A9 bound with compound...
| File | emd_50614_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of Coxsackievirus A9 bound with compound 15 (CL278) obtained by cryoEM and single-particle processing in cryoSPARC. | ||||||||||||
| Projections & Slices |
| ||||||||||||
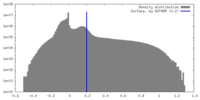
| Density Histograms |
-Half map: Half map of Coxsackievirus A9 bound with compound...
| File | emd_50614_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of Coxsackievirus A9 bound with compound 15 (CL278) obtained by cryoEM and single-particle processing in cryoSPARC. | ||||||||||||
| Projections & Slices |
| ||||||||||||
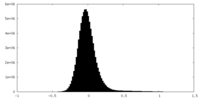
| Density Histograms |
- Sample components
Sample components
-Entire : Coxsackievirus A9
| Entire | Name:  Coxsackievirus A9 Coxsackievirus A9 |
|---|---|
| Components |
|
-Supramolecule #1: Coxsackievirus A9
| Supramolecule | Name: Coxsackievirus A9 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 Details: Coxsackievirus A9 was propagated on green monkey kidney cells and purified on a sucrose gradient. NCBI-ID: 12067 / Sci species name: Coxsackievirus A9 / Sci species strain: Griggs / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 8 MDa |
| Virus shell | Shell ID: 1 / Name: icosahedral capsid / Diameter: 300.0 Å / T number (triangulation number): 1 |
-Macromolecule #1: Capsid protein VP1
| Macromolecule | Name: Capsid protein VP1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human coxsackievirus A9 (strain Griggs) Human coxsackievirus A9 (strain Griggs) |
| Molecular weight | Theoretical: 33.86902 KDa |
| Sequence | String: GDVEEAIERA VVHVADTMRS GPSNSASVPA LTAVETGHTS QVTPSDTMQT RHVKNYHSRS ESTVENFLGR SACVYMEEYK TTDNDVNKK FVAWPINTKQ MVQMRRKLEM FTYLRFDMEV TFVITSRQDP GTTLAQDMPV LTHQIMYVPP GGPIPAKVDD Y AWQTSTNP ...String: GDVEEAIERA VVHVADTMRS GPSNSASVPA LTAVETGHTS QVTPSDTMQT RHVKNYHSRS ESTVENFLGR SACVYMEEYK TTDNDVNKK FVAWPINTKQ MVQMRRKLEM FTYLRFDMEV TFVITSRQDP GTTLAQDMPV LTHQIMYVPP GGPIPAKVDD Y AWQTSTNP SIFWTEGNAP ARMSIPFISI GNAYSNFYDG WSNFDQRGSY GYNTLNNLGH IYVRHVSGSS PHPITSTIRV YF KPKHTRA WVPRPPRLCQ YKKAFSVDFT PTPITDTRKD INTVTTVAQS RRRGDMSTLN TH UniProtKB: Genome polyprotein |
-Macromolecule #2: Capsid protein VP2
| Macromolecule | Name: Capsid protein VP2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human coxsackievirus A9 (strain Griggs) Human coxsackievirus A9 (strain Griggs) |
| Molecular weight | Theoretical: 27.791363 KDa |
| Sequence | String: SDRVRSITLG NSTITTQECA NVVVGYGRWP TYLRDDEATA EDQPTQPDVA TCRFYTLDSI KWEKGSVGWW WKFPEALSDM GLFGQNMQY HYLGRAGYTI HVQCNASKFH QGCLLVVCVP EAEMGGAVVG QAFSATAMAN GDKAYEFTSA TQSDQTKVQT A IHNAGMGV ...String: SDRVRSITLG NSTITTQECA NVVVGYGRWP TYLRDDEATA EDQPTQPDVA TCRFYTLDSI KWEKGSVGWW WKFPEALSDM GLFGQNMQY HYLGRAGYTI HVQCNASKFH QGCLLVVCVP EAEMGGAVVG QAFSATAMAN GDKAYEFTSA TQSDQTKVQT A IHNAGMGV GVGNLTIYPH QWINLRTNNS ATIVMPYINS VPMDNMFRHY NFTLMVIPFV KLDYADTAST YVPITVTVAP MC AEYNGLR LAQA UniProtKB: Genome polyprotein |
-Macromolecule #3: Capsid protein VP3
| Macromolecule | Name: Capsid protein VP3 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human coxsackievirus A9 (strain Griggs) Human coxsackievirus A9 (strain Griggs) |
| Molecular weight | Theoretical: 26.335072 KDa |
| Sequence | String: GLPTMNTPGS TQFLTSDDFQ SPCALPQFDV TPSMNIPGEV KNLMEIAEVD SVVPVNNVQD TTDQMEMFRI PVTINAPLQQ QVFGLRLQP GLDSVFKHTL LGEILNYYAH WSGSMKLTFV FCGSAMATGK FLIAYSPPGA NPPKTRKDAM LGTHIIWDIG L QSSCVLCV ...String: GLPTMNTPGS TQFLTSDDFQ SPCALPQFDV TPSMNIPGEV KNLMEIAEVD SVVPVNNVQD TTDQMEMFRI PVTINAPLQQ QVFGLRLQP GLDSVFKHTL LGEILNYYAH WSGSMKLTFV FCGSAMATGK FLIAYSPPGA NPPKTRKDAM LGTHIIWDIG L QSSCVLCV PWISQTHYRL VQQDEYTSAG YVTCWYQTGM IVPPGTPNSS SIMCFASACN DFSVRMLRDT PFISQDNKLQ UniProtKB: Genome polyprotein |
-Macromolecule #4: Capsid protein VP4
| Macromolecule | Name: Capsid protein VP4 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human coxsackievirus A9 (strain Griggs) Human coxsackievirus A9 (strain Griggs) |
| Molecular weight | Theoretical: 7.352039 KDa |
| Sequence | String: GAQVSTQKTG AHETSLSAAG NSIIHYTNIN YYKDAASNSA NRQDFTQDPS KFTEPVKDVM IKSLPALN UniProtKB: Genome polyprotein |
-Macromolecule #5: ~{N}-[(4-fluorophenyl)methyl]-4-[(4-methylpiperazin-1-yl)methyl]a...
| Macromolecule | Name: ~{N}-[(4-fluorophenyl)methyl]-4-[(4-methylpiperazin-1-yl)methyl]aniline type: ligand / ID: 5 / Number of copies: 1 / Formula: A1IEI |
|---|---|
| Molecular weight | Theoretical: 313.412 Da |
-Macromolecule #6: MYRISTIC ACID
| Macromolecule | Name: MYRISTIC ACID / type: ligand / ID: 6 / Number of copies: 1 / Formula: MYR |
|---|---|
| Molecular weight | Theoretical: 228.371 Da |
| Chemical component information |  ChemComp-MYR: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.4 mg/mL |
|---|---|
| Buffer | pH: 7.2 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 2 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 85 % / Chamber temperature: 295.15 K / Instrument: LEICA EM GP / Details: Sample was blotted from the front for 1.5 s.. |
| Details | Compound 15 (CL278) was solubilized in DMSO and diluted to the final concentration in PBS + 2 mM MgCl2. Purified Coxsackievirus A9 was incubated with compound 15 (CL278) for 1h at 37C after which the sample was vitrified on a semi-automatic plunger Leica EM GP on copper Quantifoil R1.2/1.3 grids with holey carbon film and 300 mesh. This sample was monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Temperature | Min: 93.15 K / Max: 103.15 K |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 1496 pixel / Digitization - Dimensions - Height: 1496 pixel / Number grids imaged: 1 / Number real images: 618 / Average exposure time: 1.0 sec. / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 2.9 µm / Calibrated defocus min: 0.4 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 150000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)