[English] 日本語
 Yorodumi
Yorodumi- EMDB-4891: Structural basis of Poxvirus transcription: transcribing and capp... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4891 | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structural basis of Poxvirus transcription: transcribing and capping complexes (nucleic acid free particle population) | ||||||||||||||||||||||||
 Map data Map data | Post-processed map. | ||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||
| Biological species |  Vaccinia virus Vaccinia virus | ||||||||||||||||||||||||
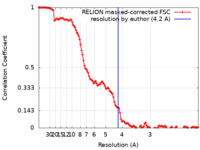
| Method | single particle reconstruction / cryo EM / Resolution: 4.2 Å | ||||||||||||||||||||||||
 Authors Authors | Hillen HS / Bartuli J / Grimm C / Dienemann C / Bedenk K / Szalay A / Fischer U / Cramer P | ||||||||||||||||||||||||
| Funding support |  Germany, 7 items Germany, 7 items
| ||||||||||||||||||||||||
 Citation Citation |  Journal: Cell / Year: 2019 Journal: Cell / Year: 2019Title: Structural Basis of Poxvirus Transcription: Transcribing and Capping Vaccinia Complexes. Authors: Hauke S Hillen / Julia Bartuli / Clemens Grimm / Christian Dienemann / Kristina Bedenk / Aladar A Szalay / Utz Fischer / Patrick Cramer /   Abstract: Poxviruses use virus-encoded multisubunit RNA polymerases (vRNAPs) and RNA-processing factors to generate mG-capped mRNAs in the host cytoplasm. In the accompanying paper, we report structures of ...Poxviruses use virus-encoded multisubunit RNA polymerases (vRNAPs) and RNA-processing factors to generate mG-capped mRNAs in the host cytoplasm. In the accompanying paper, we report structures of core and complete vRNAP complexes of the prototypic Vaccinia poxvirus (Grimm et al., 2019; in this issue of Cell). Here, we present the cryo-electron microscopy (cryo-EM) structures of Vaccinia vRNAP in the form of a transcribing elongation complex and in the form of a co-transcriptional capping complex that contains the viral capping enzyme (CE). The trifunctional CE forms two mobile modules that bind the polymerase surface around the RNA exit tunnel. RNA extends from the vRNAP active site through this tunnel and into the active site of the CE triphosphatase. Structural comparisons suggest that growing RNA triggers large-scale rearrangements on the surface of the transcription machinery during the transition from transcription initiation to RNA capping and elongation. Our structures unravel the basis for synthesis and co-transcriptional modification of poxvirus RNA. | ||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4891.map.gz emd_4891.map.gz | 10.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4891-v30.xml emd-4891-v30.xml emd-4891.xml emd-4891.xml | 20.3 KB 20.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4891_fsc.xml emd_4891_fsc.xml | 12.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_4891.png emd_4891.png | 114.1 KB | ||
| Masks |  emd_4891_msk_1.map emd_4891_msk_1.map | 149.9 MB |  Mask map Mask map | |
| Others |  emd_4891_additional.map.gz emd_4891_additional.map.gz emd_4891_additional_1.map.gz emd_4891_additional_1.map.gz emd_4891_half_map_1.map.gz emd_4891_half_map_1.map.gz emd_4891_half_map_2.map.gz emd_4891_half_map_2.map.gz | 117.8 MB 117.8 MB 118.5 MB 118.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4891 http://ftp.pdbj.org/pub/emdb/structures/EMD-4891 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4891 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4891 | HTTPS FTP |
-Validation report
| Summary document |  emd_4891_validation.pdf.gz emd_4891_validation.pdf.gz | 450.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_4891_full_validation.pdf.gz emd_4891_full_validation.pdf.gz | 449.9 KB | Display | |
| Data in XML |  emd_4891_validation.xml.gz emd_4891_validation.xml.gz | 18.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4891 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4891 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4891 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4891 | HTTPS FTP |
-Related structure data
| Related structure data |  4888C  4889C  4890C  6ricC  6ridC  6rieC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_4891.map.gz / Format: CCP4 / Size: 149.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4891.map.gz / Format: CCP4 / Size: 149.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Post-processed map. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_4891_msk_1.map emd_4891_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: 3D Refinement map.
| File | emd_4891_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3D Refinement map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: 3D Refinement map.
| File | emd_4891_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3D Refinement map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_4891_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_4891_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Vaccinia Virus DNA-dependent RNA polymerase co-transcriptional ca...
| Entire | Name: Vaccinia Virus DNA-dependent RNA polymerase co-transcriptional capping complex |
|---|---|
| Components |
|
-Supramolecule #1: Vaccinia Virus DNA-dependent RNA polymerase co-transcriptional ca...
| Supramolecule | Name: Vaccinia Virus DNA-dependent RNA polymerase co-transcriptional capping complex type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#13 |
|---|---|
| Source (natural) | Organism:  Vaccinia virus / Strain: 1h68 Vaccinia virus / Strain: 1h68 |
| Recombinant expression | Organism:  Vaccinia virus / Recombinant strain: 1h439 Vaccinia virus / Recombinant strain: 1h439 |
| Molecular weight | Theoretical: 580 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK II |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Number real images: 4958 / Average electron dose: 1.02 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)