[English] 日本語
 Yorodumi
Yorodumi- EMDB-45856: CryoEM Structure of the C-terminally truncated form of human NAD ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM Structure of the C-terminally truncated form of human NAD Kinase bound to NAD | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | catalyzes the reaction of ATP + NAD+ = ADP + H+ + NADP+ / SIGNALING PROTEIN | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationNAD+ kinase / NAD+ kinase activity / NADP+ biosynthetic process / Nicotinate metabolism / NAD+ metabolic process / phosphorylation / positive regulation of insulin secretion involved in cellular response to glucose stimulus / ATP metabolic process / ATP binding / metal ion binding / cytosol Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.34 Å | ||||||||||||||||||
 Authors Authors | Li Y / Chen Z / Mary C / Labesse G / Hoxhaj G | ||||||||||||||||||
| Funding support |  United States, United States,  France, 5 items France, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2025 Journal: Sci Adv / Year: 2025Title: Cryo-EM structure and regulation of human NAD kinase. Authors: Prakash P Praharaj / Yang Li / Charline Mary / Mona H Soflaee / Kevin Ryu / Dohun Kim / Diem H Tran / Trishna Dey / Harrison J Tom / Halie Rion / Muriel Gelin / Andrew Lemoff / Lauren G ...Authors: Prakash P Praharaj / Yang Li / Charline Mary / Mona H Soflaee / Kevin Ryu / Dohun Kim / Diem H Tran / Trishna Dey / Harrison J Tom / Halie Rion / Muriel Gelin / Andrew Lemoff / Lauren G Zacharias / João S Patricio / Thomas P Mathews / Zhe Chen / Corinne Lionne / Gerta Hoxhaj / Gilles Labesse /   Abstract: Reduced nicotinamide adenine dinucleotide phosphate (NADPH) is a crucial reducing cofactor for reductive biosynthesis and protection from oxidative stress. To fulfill their heightened anabolic and ...Reduced nicotinamide adenine dinucleotide phosphate (NADPH) is a crucial reducing cofactor for reductive biosynthesis and protection from oxidative stress. To fulfill their heightened anabolic and reductive power demands, cancer cells must boost their NADPH production. Progrowth and mitogenic protein kinases promote the activity of cytosolic NAD kinase (NADK), which produces NADP, a limiting NADPH precursor. However, the molecular architecture and mechanistic regulation of human NADK remain undescribed. Here, we report the cryo-electron microscopy structure of human NADK, both in its apo-form and in complex with its substrate NAD (nicotinamide adenine dinucleotide), revealing a tetrameric organization with distinct structural features. We discover that the amino (N)- and carboxyl (C)-terminal tails of NADK have opposing effects on its enzymatic activity and cellular NADP(H) levels. Specifically, the C-terminal region is critical for NADK activity, whereas the N-terminal region exhibits an inhibitory role. This study highlights molecular insights into the regulation of a vital enzyme governing NADP(H) production. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_45856.map.gz emd_45856.map.gz | 46.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-45856-v30.xml emd-45856-v30.xml emd-45856.xml emd-45856.xml | 19.9 KB 19.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_45856_fsc.xml emd_45856_fsc.xml | 7.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_45856.png emd_45856.png | 142.2 KB | ||
| Filedesc metadata |  emd-45856.cif.gz emd-45856.cif.gz | 6.9 KB | ||
| Others |  emd_45856_half_map_1.map.gz emd_45856_half_map_1.map.gz emd_45856_half_map_2.map.gz emd_45856_half_map_2.map.gz | 48.8 MB 48.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-45856 http://ftp.pdbj.org/pub/emdb/structures/EMD-45856 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-45856 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-45856 | HTTPS FTP |
-Validation report
| Summary document |  emd_45856_validation.pdf.gz emd_45856_validation.pdf.gz | 669.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_45856_full_validation.pdf.gz emd_45856_full_validation.pdf.gz | 669.3 KB | Display | |
| Data in XML |  emd_45856_validation.xml.gz emd_45856_validation.xml.gz | 14.9 KB | Display | |
| Data in CIF |  emd_45856_validation.cif.gz emd_45856_validation.cif.gz | 19.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-45856 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-45856 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-45856 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-45856 | HTTPS FTP |
-Related structure data
| Related structure data |  9craMC  9cr3C  9cr4C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_45856.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_45856.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0455 Å | ||||||||||||||||||||||||||||||||||||
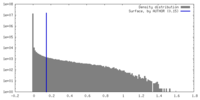
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_45856_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
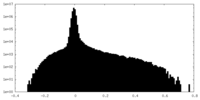
| Density Histograms |
-Half map: #1
| File | emd_45856_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
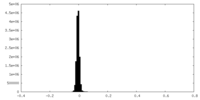
| Density Histograms |
- Sample components
Sample components
-Entire : human NAD Kinase
| Entire | Name: human NAD Kinase |
|---|---|
| Components |
|
-Supramolecule #1: human NAD Kinase
| Supramolecule | Name: human NAD Kinase / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: Tetramer of c-terminally truncated form of human NAD Kinase bound to NAD |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 49 kDa/nm |
-Macromolecule #1: NAD kinase
| Macromolecule | Name: NAD kinase / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number: NAD+ kinase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.06991 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPSPVTTFGP KATVETQDPA SVRLTWNKSP KSVLVIKKMR DASLLQPFKE LCTHLMEENM IVYVEKKVLE DPAIASDESF GAVKKKFTT FREDYDDISN QIDFIICLGG DGTLLYASSL FQGSVPPVMA FHLGSLGFLT PFSFENFQSQ VTQVIEGNAA V VLRSRLKV ...String: MPSPVTTFGP KATVETQDPA SVRLTWNKSP KSVLVIKKMR DASLLQPFKE LCTHLMEENM IVYVEKKVLE DPAIASDESF GAVKKKFTT FREDYDDISN QIDFIICLGG DGTLLYASSL FQGSVPPVMA FHLGSLGFLT PFSFENFQSQ VTQVIEGNAA V VLRSRLKV RVVKELRGKK TAVHNGLGEN GSQAAGLDMD VGKQAMQYQV LNEVVIDRGP SSYLSNVDVY LDGHLITTVQ GD GVIVSTP TGSTAYAAAA GASMIHPNVP AIMITPICPH SLSFRPIVVP AGVELKIMLS PEARNTAWVS FDGRKRQEIR HGD SISITT STYPLPSICV RDPVSDWFES LAQCLHWNVR KKQAHFELGL EHHHHHH UniProtKB: NAD kinase |
-Macromolecule #2: NICOTINAMIDE-ADENINE-DINUCLEOTIDE
| Macromolecule | Name: NICOTINAMIDE-ADENINE-DINUCLEOTIDE / type: ligand / ID: 2 / Number of copies: 4 / Formula: NAD |
|---|---|
| Molecular weight | Theoretical: 663.425 Da |
| Chemical component information |  ChemComp-NAD: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.8 / Component:
| ||||||||
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 80 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.038 kPa | ||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 278 K / Instrument: FEI VITROBOT MARK IV | ||||||||
| Details | Purified by FPLC. Monodispersed. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: TFS Selectris X / Energy filter - Slit width: 10 eV |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Number real images: 9105 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.9 µm / Nominal magnification: 165000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|---|
| Output model |  PDB-9cra: |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)