[English] 日本語
 Yorodumi
Yorodumi- EMDB-45154: Cryo-EM structure of the human P2X1 receptor in the NF449-bound i... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the human P2X1 receptor in the NF449-bound inhibited state | |||||||||
 Map data Map data | DeepEMhancer sharpened map for hP2X1 in the NF449-bound inhibited state | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Membrane Protein / Ion Channel / Ligand-gated Ion Channel / P2X Receptor / Allosteric Antagonist | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of vascular associated smooth muscle contraction / Platelet homeostasis / insemination / positive regulation of calcium ion import across plasma membrane / purinergic nucleotide receptor activity / extracellularly ATP-gated monoatomic cation channel activity / suramin binding / serotonin secretion by platelet / regulation of presynaptic cytosolic calcium ion concentration / Elevation of cytosolic Ca2+ levels ...regulation of vascular associated smooth muscle contraction / Platelet homeostasis / insemination / positive regulation of calcium ion import across plasma membrane / purinergic nucleotide receptor activity / extracellularly ATP-gated monoatomic cation channel activity / suramin binding / serotonin secretion by platelet / regulation of presynaptic cytosolic calcium ion concentration / Elevation of cytosolic Ca2+ levels / ligand-gated calcium channel activity / ceramide biosynthetic process / regulation of synaptic vesicle exocytosis / response to ATP / neuronal action potential / specific granule membrane / monoatomic cation channel activity / monoatomic ion transport / presynaptic active zone membrane / secretory granule membrane / synaptic transmission, glutamatergic / calcium ion transmembrane transport / platelet activation / regulation of blood pressure / postsynaptic membrane / membrane raft / external side of plasma membrane / glutamatergic synapse / Neutrophil degranulation / protein-containing complex binding / apoptotic process / signal transduction / protein-containing complex / ATP binding / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
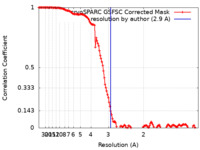
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Oken AC / Lisi NE / Ditter IA / Shi H / Mansoor SE | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Cryo-EM structures of the human P2X1 receptor reveal subtype-specific architecture and antagonism by supramolecular ligand-binding. Authors: Adam C Oken / Nicolas E Lisi / Ismayn A Ditter / Haoyuan Shi / Nadia A Nechiporuk / Steven E Mansoor /  Abstract: P2X receptors are a family of seven trimeric non-selective cation channels that are activated by extracellular ATP to play roles in the cardiovascular, neuronal, and immune systems. Although it is ...P2X receptors are a family of seven trimeric non-selective cation channels that are activated by extracellular ATP to play roles in the cardiovascular, neuronal, and immune systems. Although it is known that the P2X1 receptor subtype has increased sensitivity to ATP and fast desensitization kinetics, an underlying molecular explanation for these subtype-selective features is lacking. Here we report high-resolution cryo-EM structures of the human P2X1 receptor in the apo closed, ATP-bound desensitized, and the high-affinity antagonist NF449-bound inhibited states. The apo closed and ATP-bound desensitized state structures of human P2X1 define subtype-specific properties such as distinct pore architecture and ATP-interacting residues. The NF449-bound inhibited state structure of human P2X1 reveals that NF449 has a unique dual-ligand supramolecular binding mode at the interface of neighboring protomers, inhibiting channel activation by overlapping with the canonical P2X receptor ATP-binding site. Altogether, these data define the molecular pharmacology of the human P2X1 receptor laying the foundation for structure-based drug design. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_45154.map.gz emd_45154.map.gz | 181.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-45154-v30.xml emd-45154-v30.xml emd-45154.xml emd-45154.xml | 20.9 KB 20.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_45154_fsc.xml emd_45154_fsc.xml | 13.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_45154.png emd_45154.png | 109.6 KB | ||
| Masks |  emd_45154_msk_1.map emd_45154_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-45154.cif.gz emd-45154.cif.gz | 6.1 KB | ||
| Others |  emd_45154_additional_1.map.gz emd_45154_additional_1.map.gz emd_45154_additional_2.map.gz emd_45154_additional_2.map.gz emd_45154_half_map_1.map.gz emd_45154_half_map_1.map.gz emd_45154_half_map_2.map.gz emd_45154_half_map_2.map.gz | 202.1 MB 118.2 MB 198.8 MB 198.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-45154 http://ftp.pdbj.org/pub/emdb/structures/EMD-45154 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-45154 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-45154 | HTTPS FTP |
-Validation report
| Summary document |  emd_45154_validation.pdf.gz emd_45154_validation.pdf.gz | 784.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_45154_full_validation.pdf.gz emd_45154_full_validation.pdf.gz | 783.9 KB | Display | |
| Data in XML |  emd_45154_validation.xml.gz emd_45154_validation.xml.gz | 22.2 KB | Display | |
| Data in CIF |  emd_45154_validation.cif.gz emd_45154_validation.cif.gz | 28.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-45154 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-45154 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-45154 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-45154 | HTTPS FTP |
-Related structure data
| Related structure data |  9c2cMC  9c2aC  9c2bC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_45154.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_45154.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | DeepEMhancer sharpened map for hP2X1 in the NF449-bound inhibited state | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.6488 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_45154_msk_1.map emd_45154_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Sharpened map for hP2X1 in the NF449-bound inhibited state
| File | emd_45154_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map for hP2X1 in the NF449-bound inhibited state | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unsharpened map for hP2X1 in the NF449-bound inhibited state
| File | emd_45154_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map for hP2X1 in the NF449-bound inhibited state | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A for hP2X1 in the NF449-bound inhibited state
| File | emd_45154_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A for hP2X1 in the NF449-bound inhibited state | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B for hP2X1 in the NF449-bound inhibited state
| File | emd_45154_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B for hP2X1 in the NF449-bound inhibited state | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Membrane protein
| Entire | Name: Membrane protein |
|---|---|
| Components |
|
-Supramolecule #1: Membrane protein
| Supramolecule | Name: Membrane protein / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: P2X purinoceptor 1
| Macromolecule | Name: P2X purinoceptor 1 / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 45.038957 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MARRFQEELA AFLFEYDTPR MVLVRNKKVG VIFRLIQLVV LVYVIGWVFL YEKGYQTSSG LISSVSVKLK GLAVTQLPGL GPQVWDVAD YVFPAQGDNS FVVMTNFIVT PKQTQGYCAE HPEGGICKED SGCTPGKAKR KAQGIRTGKC VAFNDTVKTC E IFGWCPVE ...String: MARRFQEELA AFLFEYDTPR MVLVRNKKVG VIFRLIQLVV LVYVIGWVFL YEKGYQTSSG LISSVSVKLK GLAVTQLPGL GPQVWDVAD YVFPAQGDNS FVVMTNFIVT PKQTQGYCAE HPEGGICKED SGCTPGKAKR KAQGIRTGKC VAFNDTVKTC E IFGWCPVE VDDDIPRPAL LREAENFTLF IKNSISFPRF KVNRRNLVEE VNAAHMKTCL FHKTLHPLCP VFQLGYVVQE SG QNFSTLA EKGGVVGITI DWHCDLDWHV RHCRPIYEFH GLYEEKNLSP GFNFRFARHF VENGTNYRHL FKVFGIRFDI LVD GKAGKF DIIPTMTTIG SGIGIFGVAT VLCDLLLLHI LPKRHYYKQK KFKYAEDMGP GAAERDLAAT SSTLGLQENM RTS UniProtKB: P2X purinoceptor 1 |
-Macromolecule #2: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 2 / Number of copies: 9 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #3: 4,4',4'',4'''-{carbonylbis[azanediylbenzene-5,1,3-triylbis(carbon...
| Macromolecule | Name: 4,4',4'',4'''-{carbonylbis[azanediylbenzene-5,1,3-triylbis(carbonylazanediyl)]}tetra(benzene-1,3-disulfonic acid) type: ligand / ID: 3 / Number of copies: 6 / Formula: A1ALI |
|---|---|
| Molecular weight | Theoretical: 1.329236 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 22866 / Average electron dose: 45.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.4000000000000001 µm / Nominal defocus min: 0.9 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)