[English] 日本語
 Yorodumi
Yorodumi- EMDB-44650: Cryo-EM structure of Thermococcus kodakarensis FttA-dependent tra... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Thermococcus kodakarensis FttA-dependent transcription pre-termination complex containing 52 nt RNA (local refinement map) | |||||||||
 Map data Map data | local map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RNA polymerase / pre-termination complex / FttA / archaea / TRANSCRIPTION | |||||||||
| Biological species |   Thermococcus kodakarensis (archaea) Thermococcus kodakarensis (archaea) | |||||||||
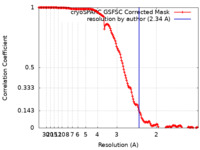
| Method | single particle reconstruction / cryo EM / Resolution: 2.34 Å | |||||||||
 Authors Authors | You L / Ebright RH | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Structural basis of archaeal FttA-dependent transcription termination. Authors: Linlin You / Chengyuan Wang / Vadim Molodtsov / Konstantin Kuznedelov / Xinyi Miao / Breanna R Wenck / Paul Ulisse / Travis J Sanders / Craig J Marshall / Emre Firlar / Jason T Kaelber / ...Authors: Linlin You / Chengyuan Wang / Vadim Molodtsov / Konstantin Kuznedelov / Xinyi Miao / Breanna R Wenck / Paul Ulisse / Travis J Sanders / Craig J Marshall / Emre Firlar / Jason T Kaelber / Thomas J Santangelo / Richard H Ebright /    Abstract: The ribonuclease FttA (also known as aCPSF and aCPSF1) mediates factor-dependent transcription termination in archaea. Here we report the structure of a Thermococcus kodakarensis transcription pre- ...The ribonuclease FttA (also known as aCPSF and aCPSF1) mediates factor-dependent transcription termination in archaea. Here we report the structure of a Thermococcus kodakarensis transcription pre-termination complex comprising FttA, Spt4, Spt5 and a transcription elongation complex (TEC). The structure shows that FttA interacts with the TEC in a manner that enables RNA to proceed directly from the TEC RNA-exit channel to the FttA catalytic centre and that enables endonucleolytic cleavage of RNA by FttA, followed by 5'→3' exonucleolytic cleavage of RNA by FttA and concomitant 5'→3' translocation of FttA on RNA, to apply mechanical force to the TEC and trigger termination. The structure further reveals that Spt5 bridges FttA and the TEC, explaining how Spt5 stimulates FttA-dependent termination. The results reveal functional analogy between bacterial and archaeal factor-dependent termination, functional homology between archaeal and eukaryotic factor-dependent termination, and fundamental mechanistic similarities in factor-dependent termination in bacteria, archaea, and eukaryotes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_44650.map.gz emd_44650.map.gz | 176.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-44650-v30.xml emd-44650-v30.xml emd-44650.xml emd-44650.xml | 17 KB 17 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_44650_fsc.xml emd_44650_fsc.xml | 14.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_44650.png emd_44650.png | 98.8 KB | ||
| Filedesc metadata |  emd-44650.cif.gz emd-44650.cif.gz | 4.4 KB | ||
| Others |  emd_44650_half_map_1.map.gz emd_44650_half_map_1.map.gz emd_44650_half_map_2.map.gz emd_44650_half_map_2.map.gz | 327.2 MB 327.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-44650 http://ftp.pdbj.org/pub/emdb/structures/EMD-44650 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44650 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44650 | HTTPS FTP |
-Validation report
| Summary document |  emd_44650_validation.pdf.gz emd_44650_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_44650_full_validation.pdf.gz emd_44650_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_44650_validation.xml.gz emd_44650_validation.xml.gz | 23.6 KB | Display | |
| Data in CIF |  emd_44650_validation.cif.gz emd_44650_validation.cif.gz | 30.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44650 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44650 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44650 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44650 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_44650.map.gz / Format: CCP4 / Size: 352.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_44650.map.gz / Format: CCP4 / Size: 352.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | local map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.73 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_44650_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_44650_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : FttA-dependent transcription termination complex containing 52 nt RNA
| Entire | Name: FttA-dependent transcription termination complex containing 52 nt RNA |
|---|---|
| Components |
|
-Supramolecule #1: FttA-dependent transcription termination complex containing 52 nt RNA
| Supramolecule | Name: FttA-dependent transcription termination complex containing 52 nt RNA type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#17 |
|---|---|
| Source (natural) | Organism:   Thermococcus kodakarensis (archaea) Thermococcus kodakarensis (archaea) |
| Molecular weight | Theoretical: 556 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 300 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 48.33 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)