[English] 日本語
 Yorodumi
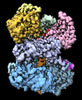
Yorodumi- EMDB-44358: Human polymerase epsilon bound to PCNA and DNA in the nucleotide ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human polymerase epsilon bound to PCNA and DNA in the nucleotide bound state | |||||||||
 Map data Map data | Final sharpen cryo-em by cryosparc | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DNA polymerase / DNA / DNA Binding Protein-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of deoxyribonuclease activity / dinucleotide insertion or deletion binding / epsilon DNA polymerase complex / PCNA-p21 complex / mitotic telomere maintenance via semi-conservative replication / purine-specific mismatch base pair DNA N-glycosylase activity / MutLalpha complex binding / positive regulation of DNA-directed DNA polymerase activity / nuclear lamina / Polymerase switching ...positive regulation of deoxyribonuclease activity / dinucleotide insertion or deletion binding / epsilon DNA polymerase complex / PCNA-p21 complex / mitotic telomere maintenance via semi-conservative replication / purine-specific mismatch base pair DNA N-glycosylase activity / MutLalpha complex binding / positive regulation of DNA-directed DNA polymerase activity / nuclear lamina / Polymerase switching / Telomere C-strand (Lagging Strand) Synthesis / Processive synthesis on the lagging strand / PCNA complex / Removal of the Flap Intermediate / Processive synthesis on the C-strand of the telomere / Mismatch repair (MMR) directed by MSH2:MSH3 (MutSbeta) / Polymerase switching on the C-strand of the telomere / Mismatch repair (MMR) directed by MSH2:MSH6 (MutSalpha) / Removal of the Flap Intermediate from the C-strand / Transcription of E2F targets under negative control by DREAM complex / replisome / response to L-glutamate / histone acetyltransferase binding / DNA synthesis involved in DNA repair / leading strand elongation / DNA polymerase processivity factor activity / G1/S-Specific Transcription / response to dexamethasone / replication fork processing / nuclear replication fork / SUMOylation of DNA replication proteins / PCNA-Dependent Long Patch Base Excision Repair / estrous cycle / mismatch repair / translesion synthesis / response to cadmium ion / DNA polymerase binding / cyclin-dependent protein kinase holoenzyme complex / epithelial cell differentiation / base-excision repair, gap-filling / positive regulation of DNA repair / Translesion synthesis by REV1 / Translesion synthesis by POLK / TP53 Regulates Transcription of Genes Involved in G2 Cell Cycle Arrest / Translesion synthesis by POLI / Gap-filling DNA repair synthesis and ligation in GG-NER / positive regulation of DNA replication / male germ cell nucleus / replication fork / nuclear estrogen receptor binding / liver regeneration / Recognition of DNA damage by PCNA-containing replication complex / Termination of translesion DNA synthesis / Translesion Synthesis by POLH / HDR through Homologous Recombination (HRR) / Dual Incision in GG-NER / DNA-templated DNA replication / receptor tyrosine kinase binding / cellular response to hydrogen peroxide / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / cellular response to UV / cellular response to xenobiotic stimulus / E3 ubiquitin ligases ubiquitinate target proteins / response to estradiol / heart development / 4 iron, 4 sulfur cluster binding / damaged DNA binding / DNA-directed DNA polymerase / chromosome, telomeric region / DNA-directed DNA polymerase activity / nuclear body / nucleotide binding / centrosome / chromatin binding / protein-containing complex binding / chromatin / negative regulation of transcription by RNA polymerase II / enzyme binding / DNA binding / extracellular exosome / zinc ion binding / nucleoplasm / identical protein binding / nucleus Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / DNA molecule (others) Homo sapiens (human) / DNA molecule (others) | |||||||||
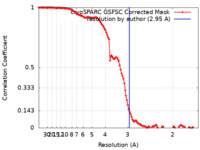
| Method | single particle reconstruction / cryo EM / Resolution: 2.95 Å | |||||||||
 Authors Authors | Wang F / He Q / Li H | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structures of the human leading strand Polε-PCNA holoenzyme. Authors: Qing He / Feng Wang / Nina Y Yao / Michael E O'Donnell / Huilin Li /  Abstract: In eukaryotes, the leading strand DNA is synthesized by Polε and the lagging strand by Polδ. These replicative polymerases have higher processivity when paired with the DNA clamp PCNA. While the ...In eukaryotes, the leading strand DNA is synthesized by Polε and the lagging strand by Polδ. These replicative polymerases have higher processivity when paired with the DNA clamp PCNA. While the structure of the yeast Polε catalytic domain has been determined, how Polε interacts with PCNA is unknown in any eukaryote, human or yeast. Here we report two cryo-EM structures of human Polε-PCNA-DNA complex, one in an incoming nucleotide bound state and the other in a nucleotide exchange state. The structures reveal an unexpected three-point interface between the Polε catalytic domain and PCNA, with the conserved PIP (PCNA interacting peptide)-motif, the unique P-domain, and the thumb domain each interacting with a different protomer of the PCNA trimer. We propose that the multi-point interface prevents other PIP-containing factors from recruiting to PCNA while PCNA functions with Polε. Comparison of the two states reveals that the finger domain pivots around the [4Fe-4S] cluster-containing tip of the P-domain to regulate nucleotide exchange and incoming nucleotide binding. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_44358.map.gz emd_44358.map.gz | 168 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-44358-v30.xml emd-44358-v30.xml emd-44358.xml emd-44358.xml | 23.9 KB 23.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_44358_fsc.xml emd_44358_fsc.xml | 11.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_44358.png emd_44358.png | 176.6 KB | ||
| Filedesc metadata |  emd-44358.cif.gz emd-44358.cif.gz | 8.2 KB | ||
| Others |  emd_44358_additional_1.map.gz emd_44358_additional_1.map.gz emd_44358_half_map_1.map.gz emd_44358_half_map_1.map.gz emd_44358_half_map_2.map.gz emd_44358_half_map_2.map.gz | 89.3 MB 165.3 MB 165.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-44358 http://ftp.pdbj.org/pub/emdb/structures/EMD-44358 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44358 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44358 | HTTPS FTP |
-Validation report
| Summary document |  emd_44358_validation.pdf.gz emd_44358_validation.pdf.gz | 893 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_44358_full_validation.pdf.gz emd_44358_full_validation.pdf.gz | 892.5 KB | Display | |
| Data in XML |  emd_44358_validation.xml.gz emd_44358_validation.xml.gz | 20.5 KB | Display | |
| Data in CIF |  emd_44358_validation.cif.gz emd_44358_validation.cif.gz | 26.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44358 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44358 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44358 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44358 | HTTPS FTP |
-Related structure data
| Related structure data |  9b8tMC  9b8sC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_44358.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_44358.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Final sharpen cryo-em by cryosparc | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.828 Å | ||||||||||||||||||||||||||||||||||||
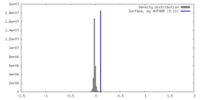
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Final unsharpened EM map
| File | emd_44358_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Final unsharpened EM map | ||||||||||||
| Projections & Slices |
| ||||||||||||
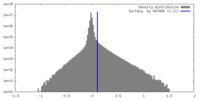
| Density Histograms |
-Half map: half map A
| File | emd_44358_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
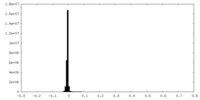
| Density Histograms |
-Half map: half map B
| File | emd_44358_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
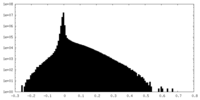
| Density Histograms |
- Sample components
Sample components
-Entire : The DNA bound Pol epsilon and PCNA complex
| Entire | Name: The DNA bound Pol epsilon and PCNA complex |
|---|---|
| Components |
|
-Supramolecule #1: The DNA bound Pol epsilon and PCNA complex
| Supramolecule | Name: The DNA bound Pol epsilon and PCNA complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 450 KDa |
-Macromolecule #1: DNA polymerase epsilon catalytic subunit
| Macromolecule | Name: DNA polymerase epsilon catalytic subunit / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: DNA-directed DNA polymerase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 261.782266 KDa |
| Recombinant expression | Organism: Insect cell expression vector pTIE1 (others) |
| Sequence | String: MSLRSGGRRR ADPGADGEAS RDDGATSSVS ALKRLERSQW TDKMDLRFGF ERLKEPGEKT GWLINMHPTE ILDEDKRLGS AVDYYFIQD DGSRFKVALP YKPYFYIATR KGCEREVSSF LSKKFQGKIA KVETVPKEDL DLPNHLVGLK RNYIRLSFHT V EDLVKVRK ...String: MSLRSGGRRR ADPGADGEAS RDDGATSSVS ALKRLERSQW TDKMDLRFGF ERLKEPGEKT GWLINMHPTE ILDEDKRLGS AVDYYFIQD DGSRFKVALP YKPYFYIATR KGCEREVSSF LSKKFQGKIA KVETVPKEDL DLPNHLVGLK RNYIRLSFHT V EDLVKVRK EISPAVKKNR EQDHASDAYT ALLSSVLQRG GVITDEEETS KKIADQLDNI VDMREYDVPY HIRLSIDLKI HV AHWYNVR YRGNAFPVEI TRRDDLVERP DPVVLAFAIA TTKLPLKFPD AETDQIMMIS YMIDGQGYLI TNREIVSEDI EDF EFTPKP EYEGPFCVFN EPDEAHLIQR WFEHVQETKP TIMVTYNGDF FDWPFVEARA AVHGLSMQQE IGFQKDSQGE YKAP QCIHM DCLRWVKRDS YLPVGSHNLK AAAKAKLGYD PVELDPEDMC RMATEQPQTL ATYSVSDAVA TYYLYMKYVH PFIFA LCTI IPMEPDEVLR KGSGTLCEAL LMVQAFHANI IFPNKQEQEF NKLTDDGHVL DSETYVGGHV EALESGVFRS DIPCRF RMN PAAFDFLLQR VEKTLRHALE EEEKVPVEQV TNFEEVCDEI KSKLASLKDV PSRIECPLIY HLDVGAMYPN IILTNRL QP SAMVDEATCA ACDFNKPGAN CQRKMAWQWR GEFMPASRSE YHRIQHQLES EKFPPLFPEG PARAFHELSR EEQAKYEK R RLADYCRKAY KKIHITKVEE RLTTICQREN SFYVDTVRAF RDRRYEFKGL HKVWKKKLSA AVEVGDAAEV KRCKNMEVL YDSLQLAHKC ILNSFYGYVM RKGARWYSME MAGIVCFTGA NIITQARELI EQIGRPLELD TDGIWCVLPN SFPENFVFKT TNVKKPKVT ISYPGAMLNI MVKEGFTNDQ YQELAEPSSL TYVTRSENSI FFEVDGPYLA MILPASKEEG KKLKKRYAVF N EDGSLAEL KGFEVKRRGE LQLIKIFQSS VFEAFLKGST LEEVYGSVAK VADYWLDVLY SKAANMPDSE LFELISENRS MS RKLEDYG EQKSTSISTA KRLAEFLGDQ MVKDAGLSCR YIISRKPEGS PVTERAIPLA IFQAEPTVRK HFLRKWLKSS SLQ DFDIRA ILDWDYYIER LGSAIQKIIT IPAALQQVKN PVPRVKHPDW LHKKLLEKND VYKQKKISEL FTLEGRRQVT MAEA SEDSP RPSAPDMEDF GLVKLPHPAA PVTVKRKRVL WESQEESQDL TPTVPWQEIL GQPPALGTSQ EEWLVWLRFH KKKWQ LQAR QRLARRKRQR LESAEGVLRP GAIRDGPATG LGSFLRRTAR SILDLPWQIV QISETSQAGL FRLWALVGSD LHCIRL SIP RVFYVNQRVA KAEEGASYRK VNRVLPRSNM VYNLYEYSVP EDMYQEHINE INAELSAPDI EGVYETQVPL LFRALVH LG CVCVVNKQLV RHLSGWEAET FALEHLEMRS LAQFSYLEPG SIRHIYLYHH AQAHKALFGI FIPSQRRASV FVLDTVRT D QMPSLGALYS AEHGLLLEKV GPELLPPPKH TFEVRAETDL KTICRAIQRF LLAYKEERRG PTLIAVQSSW ELKRLASEI PVLEEFPLVP ICVADKINYG VLDWQRHGAR RMIRHYLNLD TCLSQAFEMS RYFHIPIGNL PEDISTFGSD LFFARHLQRH NHLLWLSPT ARPDLGGKEA DDNCLVMEFD DQATVEINSS GCYSTVCVEL DLQNLAVNTI LQSHHVNDME GADSMGISFD V IQQASLED MITGGQAASA PASYDETALC SNTFRILKSM VVGWVKEITQ YHNIYADNQV MHFYRWLRSP SSLLHDPALH RT LHNMMKK LFLQLIAEFK RLGSSVIYAN FNRIILCTKK RRVEDAIAYV EYITSSIHSK ETFHSLTISF SRCWEFLLWM DPS NYGGIK GKVSSRIHCG LQDSQKAGGA EDEQENEDDE EERDGEEEEE AEESNVEDLL ENNWNILQFL PQAASCQNYF LMIV SAYIV AVYHCMKDGL RRSAPGSTPV RRRGASQLSQ EAEGAVGALP GMITFSQDYV ANELTQSFFT ITQKIQKKVT GSRNS TELS EMFPVLPGSH LLLNNPALEF IKYVCKVLSL DTNITNQVNK LNRDLLRLVD VGEFSEEAQF RDPCRSYVLP EVICRS CNF CRDLDLCKDS SFSEDGAVLP QWLCSNCQAP YDSSAIEMTL VEVLQKKLMA FTLQDLVCLK CRGVKETSMP VYCTCAG DF ALTIHTQVFM EQIGIFRNIA QHYGMSYLLE TLEWLLQKNP QLGH UniProtKB: DNA polymerase epsilon catalytic subunit |
-Macromolecule #2: Proliferating cell nuclear antigen
| Macromolecule | Name: Proliferating cell nuclear antigen / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 28.795752 KDa |
| Recombinant expression | Organism: Insect cell expression vector pTIE1 (others) |
| Sequence | String: MFEARLVQGS ILKKVLEALK DLINEACWDI SSSGVNLQSM DSSHVSLVQL TLRSEGFDTY RCDRNLAMGV NLTSMSKILK CAGNEDIIT LRAEDNADTL ALVFEAPNQE KVSDYEMKLM DLDVEQLGIP EQEYSCVVKM PSGEFARICR DLSHIGDAVV I SCAKDGVK ...String: MFEARLVQGS ILKKVLEALK DLINEACWDI SSSGVNLQSM DSSHVSLVQL TLRSEGFDTY RCDRNLAMGV NLTSMSKILK CAGNEDIIT LRAEDNADTL ALVFEAPNQE KVSDYEMKLM DLDVEQLGIP EQEYSCVVKM PSGEFARICR DLSHIGDAVV I SCAKDGVK FSASGELGNG NIKLSQTSNV DKEEEAVTIE MNEPVQLTFA LRYLNFFTKA TPLSSTVTLS MSADVPLVVE YK IADMGHL KYYLAPKIED EEGS UniProtKB: Proliferating cell nuclear antigen |
-Macromolecule #3: Primer DNA
| Macromolecule | Name: Primer DNA / type: dna / ID: 3 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: DNA molecule (others) |
| Molecular weight | Theoretical: 10.823965 KDa |
| Sequence | String: (DT)(DG)(DA)(DG)(DG)(DT)(DT)(DC)(DA)(DG) (DC)(DA)(DA)(DG)(DG)(DT)(DG)(DA)(DT)(DG) (DC)(DT)(DT)(DT)(DA)(DG)(DA)(DT)(DT) (DT)(DT)(DT)(DC)(DA)(DC) |
-Macromolecule #4: Template DNA
| Macromolecule | Name: Template DNA / type: dna / ID: 4 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: DNA molecule (others) |
| Molecular weight | Theoretical: 18.13668 KDa |
| Sequence | String: (DG)(DC)(DC)(DA)(DC)(DG)(DC)(DT)(DG)(DA) (DG)(DA)(DG)(DC)(DC)(DA)(DG)(DC)(DA)(DG) (DC)(DA)(DA)(DA)(DG)(DT)(DG)(DA)(DA) (DA)(DA)(DA)(DT)(DC)(DT)(DA)(DA)(DA)(DG) (DC) (DA)(DT)(DC)(DA)(DC)(DC) ...String: (DG)(DC)(DC)(DA)(DC)(DG)(DC)(DT)(DG)(DA) (DG)(DA)(DG)(DC)(DC)(DA)(DG)(DC)(DA)(DG) (DC)(DA)(DA)(DA)(DG)(DT)(DG)(DA)(DA) (DA)(DA)(DA)(DT)(DC)(DT)(DA)(DA)(DA)(DG) (DC) (DA)(DT)(DC)(DA)(DC)(DC)(DT)(DT) (DG)(DC)(DT)(DG)(DA)(DA)(DC)(DC)(DT)(DC) (DA) |
-Macromolecule #5: THYMIDINE-5'-TRIPHOSPHATE
| Macromolecule | Name: THYMIDINE-5'-TRIPHOSPHATE / type: ligand / ID: 5 / Number of copies: 1 / Formula: TTP |
|---|---|
| Molecular weight | Theoretical: 482.168 Da |
| Chemical component information |  ChemComp-TTP: |
-Macromolecule #6: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 6 / Number of copies: 1 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #7: IRON/SULFUR CLUSTER
| Macromolecule | Name: IRON/SULFUR CLUSTER / type: ligand / ID: 7 / Number of copies: 1 / Formula: SF4 |
|---|---|
| Molecular weight | Theoretical: 351.64 Da |
| Chemical component information |  ChemComp-FS1: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.75 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: OTHER | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 280 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-9b8t: |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)