[English] 日本語
 Yorodumi
Yorodumi- EMDB-41189: Baseplate of Nexin-dynein regulatory complex from Tetrahymena the... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Baseplate of Nexin-dynein regulatory complex from Tetrahymena thermophila | |||||||||
 Map data Map data | The baseplate domain of N-DRC on doublet microtubule in Tetrahymena thermophila cilia | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | complex / Cilia / axoneme / nexin-dynein regulatory complex / PROTEIN BINDING | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of cilium movement / axonemal dynein complex assembly / epithelial cilium movement involved in determination of left/right asymmetry / inner dynein arm assembly / cilium-dependent cell motility / regulation of cilium beat frequency involved in ciliary motility / axoneme assembly / axonemal dynein complex / flagellated sperm motility / motile cilium ...regulation of cilium movement / axonemal dynein complex assembly / epithelial cilium movement involved in determination of left/right asymmetry / inner dynein arm assembly / cilium-dependent cell motility / regulation of cilium beat frequency involved in ciliary motility / axoneme assembly / axonemal dynein complex / flagellated sperm motility / motile cilium / positive regulation of cell motility / axoneme / cilium assembly / ciliary basal body / cell projection / cell motility / small GTPase binding / cilium / microtubule binding / microtubule / cytoskeleton / Golgi apparatus / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
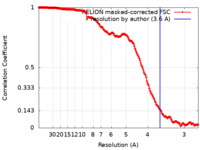
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Ghanaeian AG / Black CS / Yang SK / Bui KH | |||||||||
| Funding support |  Canada, 2 items Canada, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Integrated modeling of the Nexin-dynein regulatory complex reveals its regulatory mechanism. Authors: Avrin Ghanaeian / Sumita Majhi / Caitlyn L McCafferty / Babak Nami / Corbin S Black / Shun Kai Yang / Thibault Legal / Ophelia Papoulas / Martyna Janowska / Melissa Valente-Paterno / Edward ...Authors: Avrin Ghanaeian / Sumita Majhi / Caitlyn L McCafferty / Babak Nami / Corbin S Black / Shun Kai Yang / Thibault Legal / Ophelia Papoulas / Martyna Janowska / Melissa Valente-Paterno / Edward M Marcotte / Dorota Wloga / Khanh Huy Bui /    Abstract: Cilia are hairlike protrusions that project from the surface of eukaryotic cells and play key roles in cell signaling and motility. Ciliary motility is regulated by the conserved nexin-dynein ...Cilia are hairlike protrusions that project from the surface of eukaryotic cells and play key roles in cell signaling and motility. Ciliary motility is regulated by the conserved nexin-dynein regulatory complex (N-DRC), which links adjacent doublet microtubules and regulates and coordinates the activity of outer doublet complexes. Despite its critical role in cilia motility, the assembly and molecular basis of the regulatory mechanism are poorly understood. Here, using cryo-electron microscopy in conjunction with biochemical cross-linking and integrative modeling, we localize 12 DRC subunits in the N-DRC structure of Tetrahymena thermophila. We also find that the CCDC96/113 complex is in close contact with the DRC9/10 in the linker region. In addition, we reveal that the N-DRC is associated with a network of coiled-coil proteins that most likely mediates N-DRC regulatory activity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41189.map.gz emd_41189.map.gz | 466.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41189-v30.xml emd-41189-v30.xml emd-41189.xml emd-41189.xml | 28.2 KB 28.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_41189_fsc.xml emd_41189_fsc.xml | 18 KB | Display |  FSC data file FSC data file |
| Images |  emd_41189.png emd_41189.png | 92.7 KB | ||
| Masks |  emd_41189_msk_1.map emd_41189_msk_1.map | 512 MB |  Mask map Mask map | |
| Others |  emd_41189_half_map_1.map.gz emd_41189_half_map_1.map.gz emd_41189_half_map_2.map.gz emd_41189_half_map_2.map.gz | 412.6 MB 412.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41189 http://ftp.pdbj.org/pub/emdb/structures/EMD-41189 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41189 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41189 | HTTPS FTP |
-Validation report
| Summary document |  emd_41189_validation.pdf.gz emd_41189_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_41189_full_validation.pdf.gz emd_41189_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_41189_validation.xml.gz emd_41189_validation.xml.gz | 25.4 KB | Display | |
| Data in CIF |  emd_41189_validation.cif.gz emd_41189_validation.cif.gz | 33.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41189 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41189 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41189 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41189 | HTTPS FTP |
-Related structure data
| Related structure data |  8tekMC  8th8C  8tidC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_41189.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41189.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The baseplate domain of N-DRC on doublet microtubule in Tetrahymena thermophila cilia | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.37 Å | ||||||||||||||||||||
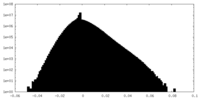
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_41189_msk_1.map emd_41189_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
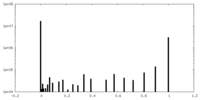
| Density Histograms |
-Half map: The half map of baseplate domain of N-DRC...
| File | emd_41189_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The half map of baseplate domain of N-DRC on doublet microtubule in Tetrahymena thermophila cilia | ||||||||||||
| Projections & Slices |
| ||||||||||||
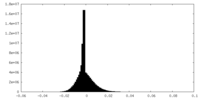
| Density Histograms |
-Half map: The half map of baseplate domain of N-DRC...
| File | emd_41189_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The half map of baseplate domain of N-DRC on doublet microtubule in Tetrahymena thermophila cilia | ||||||||||||
| Projections & Slices |
| ||||||||||||
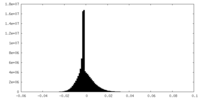
| Density Histograms |
- Sample components
Sample components
+Entire : Baseplate of Nexin-dynein regulatory complex from Tetrahymena the...
+Supramolecule #1: Baseplate of Nexin-dynein regulatory complex from Tetrahymena the...
+Macromolecule #1: Dynein regulatory complex protein 1/2 N-terminal domain-containin...
+Macromolecule #2: Coiled-coil protein, putative
+Macromolecule #3: Growth-arrest-specific microtubule-binding protein
+Macromolecule #4: Growth-arrest-specific microtubule-binding protein
+Macromolecule #5: Cilia- and flagella-associated protein 91
+Macromolecule #6: Flagella associated protein
+Macromolecule #7: Coiled-coil protein, putative
+Macromolecule #8: DUF4201 domain-containing protein
+Macromolecule #9: Flagellar associated protein
+Macromolecule #10: CFAP20
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 45.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



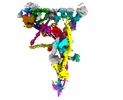

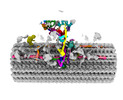



 Z
Z Y
Y X
X