[English] 日本語
 Yorodumi
Yorodumi- EMDB-39871: Cryo-EM structure of Phytanoyl-CoA-bound human very long-chain fa... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Phytanoyl-CoA-bound human very long-chain fatty acid ABC transporter ABCD3 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | very long-chain fatty / Peroxisome / ABC transporter / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationphytanic acid metabolic process / long-chain fatty acid import into peroxisome / very long-chain fatty acid catabolic process / Class I peroxisomal membrane protein import / very long-chain fatty acid metabolic process / peroxisome organization / fatty acyl-CoA hydrolase activity / ABC transporters in lipid homeostasis / bile acid biosynthetic process / Hydrolases; Acting on ester bonds; Thioester hydrolases ...phytanic acid metabolic process / long-chain fatty acid import into peroxisome / very long-chain fatty acid catabolic process / Class I peroxisomal membrane protein import / very long-chain fatty acid metabolic process / peroxisome organization / fatty acyl-CoA hydrolase activity / ABC transporters in lipid homeostasis / bile acid biosynthetic process / Hydrolases; Acting on ester bonds; Thioester hydrolases / Translocases; Catalysing the translocation of other compounds; Linked to the hydrolysis of a nucleoside triphosphate / peroxisomal membrane / bile acid and bile salt transport / long-chain fatty acid transmembrane transporter activity / fatty acid beta-oxidation / RHOC GTPase cycle / peroxisomal matrix / ATPase-coupled transmembrane transporter activity / RHOA GTPase cycle / ABC-type transporter activity / response to organic cyclic compound / fatty acid biosynthetic process / peroxisome / response to xenobiotic stimulus / intracellular membrane-bounded organelle / protein homodimerization activity / ATP hydrolysis activity / mitochondrion / ATP binding / membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.96 Å | |||||||||
 Authors Authors | Li Y / Chen YX / Zhou CZ / Hou WT | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Structural insights into human ABCD3-mediated peroxisomal acyl-CoA translocation Authors: Yang L / Wen TH | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_39871.map.gz emd_39871.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-39871-v30.xml emd-39871-v30.xml emd-39871.xml emd-39871.xml | 16.3 KB 16.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_39871.png emd_39871.png | 50.6 KB | ||
| Filedesc metadata |  emd-39871.cif.gz emd-39871.cif.gz | 6 KB | ||
| Others |  emd_39871_additional_1.map.gz emd_39871_additional_1.map.gz emd_39871_half_map_1.map.gz emd_39871_half_map_1.map.gz emd_39871_half_map_2.map.gz emd_39871_half_map_2.map.gz | 31.8 MB 59.2 MB 59.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-39871 http://ftp.pdbj.org/pub/emdb/structures/EMD-39871 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39871 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39871 | HTTPS FTP |
-Validation report
| Summary document |  emd_39871_validation.pdf.gz emd_39871_validation.pdf.gz | 804.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_39871_full_validation.pdf.gz emd_39871_full_validation.pdf.gz | 804.3 KB | Display | |
| Data in XML |  emd_39871_validation.xml.gz emd_39871_validation.xml.gz | 12.4 KB | Display | |
| Data in CIF |  emd_39871_validation.cif.gz emd_39871_validation.cif.gz | 14.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39871 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39871 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39871 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39871 | HTTPS FTP |
-Related structure data
| Related structure data |  8z9xMC  8z0fC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_39871.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_39871.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||
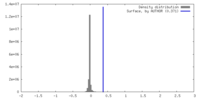
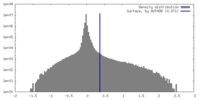
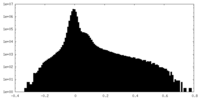
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_39871_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
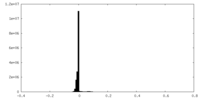
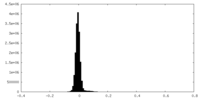
| Density Histograms |
-Half map: #2
| File | emd_39871_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
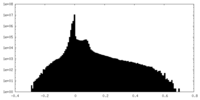
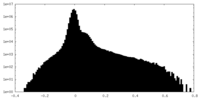
| Density Histograms |
-Half map: #1
| File | emd_39871_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
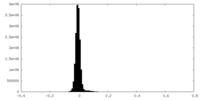
| Density Histograms |
- Sample components
Sample components
-Entire : Phytanoyl-CoA-bound human peroxisomal ABCD3
| Entire | Name: Phytanoyl-CoA-bound human peroxisomal ABCD3 |
|---|---|
| Components |
|
-Supramolecule #1: Phytanoyl-CoA-bound human peroxisomal ABCD3
| Supramolecule | Name: Phytanoyl-CoA-bound human peroxisomal ABCD3 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 140 kDa/nm |
-Macromolecule #1: ATP-binding cassette sub-family D member 3
| Macromolecule | Name: ATP-binding cassette sub-family D member 3 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO EC number: Hydrolases; Acting on ester bonds; Thioester hydrolases |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 76.67918 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDYKDDDDKA VFSKLQLLGQ AIPPKQYAPG VVGMLAVFAL IKLYKQDIRG TKHLVAKTKE GKKERAVVDK VFFSRLIQIL KIMVPRTFC KETGYLVLIA VMLVSRTYCD VWMIQNGTLI ESGIIGRSRK DFKRYLLNFI AAMPLISLVN NFLKYGLNEL K LCFRVRLT ...String: MDYKDDDDKA VFSKLQLLGQ AIPPKQYAPG VVGMLAVFAL IKLYKQDIRG TKHLVAKTKE GKKERAVVDK VFFSRLIQIL KIMVPRTFC KETGYLVLIA VMLVSRTYCD VWMIQNGTLI ESGIIGRSRK DFKRYLLNFI AAMPLISLVN NFLKYGLNEL K LCFRVRLT KYLYEEYLQA FTYYKMGNLD NRIANPDQLL TQDVEKFCNS VVDLYSNLSK PFLDIVLYIF KLTSAIGAQG PA SMMAYLV VSGLFLTRLR RPIGKMTITE QKYEGEYRYV NSRLITNSEE IAFYNGNKRE KQTVHSVFRK LVEHLHNFIL FRF SMGFID SIIAKYLATV VGYLVVSRPF LDLSHPRHLK STHSELLEDY YQSGRMLLRM SQALGRIVLA GREMTRLAGF TARI TELMQ VLKDLNHGKY ERTMVSQQEK GIEGVQVIPL IPGAGEIIIA DNIIKFDHVP LATPNGDVLI RDLNFEVRSG ANVLI CGPN GCGKSSLFRV LGELWPLFGG RLTKPERGKL FYVPQRPYMT LGTLRDQVIY PDGREDQKRK GISDLVLKEY LDNVQL GHI LEREGGWDSV QDWMDVLSGG EKQRMAMARL FYHKPQFAIL DECTSAVSVD VEGYIYSHCR KVGITLFTVS HRKSLWK HH EYYLHMDGRG NYEFKQITED TVEFGS UniProtKB: ATP-binding cassette sub-family D member 3 |
-Macromolecule #2: phytanoyl-CoA
| Macromolecule | Name: phytanoyl-CoA / type: ligand / ID: 2 / Number of copies: 2 / Formula: A1L1A |
|---|---|
| Molecular weight | Theoretical: 1.062049 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 10 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 54.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.5 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)