+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the channelrhodopsin GtCCR4 | |||||||||||||||||||||||||||
 Map data Map data | ||||||||||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||||||||
 Keywords Keywords | Microbial rhodopsin / MEMBRANE PROTEIN | |||||||||||||||||||||||||||
| Function / homology | Bacteriorhodopsin-like protein / Archaeal/bacterial/fungal rhodopsins / Bacteriorhodopsin-like protein / plasma membrane / Cation channel rhodopsin 4 Function and homology information Function and homology information | |||||||||||||||||||||||||||
| Biological species |  | |||||||||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.71 Å | |||||||||||||||||||||||||||
 Authors Authors | Tanaka T / Iida W / Sano FK / Oda K / Shihoya W / Nureki O | |||||||||||||||||||||||||||
| Funding support |  Japan, 8 items Japan, 8 items
| |||||||||||||||||||||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2024 Journal: Mol Cell / Year: 2024Title: The high-light-sensitivity mechanism and optogenetic properties of the bacteriorhodopsin-like channelrhodopsin GtCCR4. Authors: Tatsuki Tanaka / Shoko Hososhima / Yo Yamashita / Teppei Sugimoto / Toshiki Nakamura / Shunta Shigemura / Wataru Iida / Fumiya K Sano / Kazumasa Oda / Takayuki Uchihashi / Kota Katayama / ...Authors: Tatsuki Tanaka / Shoko Hososhima / Yo Yamashita / Teppei Sugimoto / Toshiki Nakamura / Shunta Shigemura / Wataru Iida / Fumiya K Sano / Kazumasa Oda / Takayuki Uchihashi / Kota Katayama / Yuji Furutani / Satoshi P Tsunoda / Wataru Shihoya / Hideki Kandori / Osamu Nureki /  Abstract: Channelrhodopsins are microbial light-gated ion channels that can control the firing of neurons in response to light. Among several cation channelrhodopsins identified in Guillardia theta (GtCCRs), ...Channelrhodopsins are microbial light-gated ion channels that can control the firing of neurons in response to light. Among several cation channelrhodopsins identified in Guillardia theta (GtCCRs), GtCCR4 has higher light sensitivity than typical channelrhodopsins. Furthermore, GtCCR4 shows superior properties as an optogenetic tool, such as minimal desensitization. Our structural analyses of GtCCR2 and GtCCR4 revealed that GtCCR4 has an outwardly bent transmembrane helix, resembling the conformation of activated G-protein-coupled receptors. Spectroscopic and electrophysiological comparisons suggested that this helix bend in GtCCR4 omits channel recovery time and contributes to high light sensitivity. An electrophysiological comparison of GtCCR4 and the well-characterized optogenetic tool ChRmine demonstrated that GtCCR4 has superior current continuity and action-potential spike generation with less invasiveness in neurons. We also identified highly active mutants of GtCCR4. These results shed light on the diverse structures and dynamics of microbial rhodopsins and demonstrate the strong optogenetic potential of GtCCR4. | |||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_39199.map.gz emd_39199.map.gz | 3.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-39199-v30.xml emd-39199-v30.xml emd-39199.xml emd-39199.xml | 20.5 KB 20.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_39199.png emd_39199.png | 102 KB | ||
| Filedesc metadata |  emd-39199.cif.gz emd-39199.cif.gz | 6.4 KB | ||
| Others |  emd_39199_half_map_1.map.gz emd_39199_half_map_1.map.gz emd_39199_half_map_2.map.gz emd_39199_half_map_2.map.gz | 77.6 MB 77.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-39199 http://ftp.pdbj.org/pub/emdb/structures/EMD-39199 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39199 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39199 | HTTPS FTP |
-Validation report
| Summary document |  emd_39199_validation.pdf.gz emd_39199_validation.pdf.gz | 825 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_39199_full_validation.pdf.gz emd_39199_full_validation.pdf.gz | 824.6 KB | Display | |
| Data in XML |  emd_39199_validation.xml.gz emd_39199_validation.xml.gz | 11.7 KB | Display | |
| Data in CIF |  emd_39199_validation.cif.gz emd_39199_validation.cif.gz | 13 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39199 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39199 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39199 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39199 | HTTPS FTP |
-Related structure data
| Related structure data |  8yelMC  8yejC  8yekC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_39199.map.gz / Format: CCP4 / Size: 4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_39199.map.gz / Format: CCP4 / Size: 4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
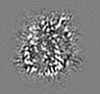
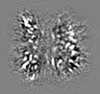
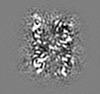
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.94857 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : GtCCR4
| Entire | Name: GtCCR4 |
|---|---|
| Components |
|
-Supramolecule #1: GtCCR4
| Supramolecule | Name: GtCCR4 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Cation channel rhodopsin 4
| Macromolecule | Name: Cation channel rhodopsin 4 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 45.933734 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDAMGTTSA PSLSDPNWQY GMGGWNNPRL PNFNLHDPTV IGVDWLGFLC LLGASLALMY KLMSFKGPD GDQEFFVGYR EEKCLSIYVN LIAAITYWGR ICAHFNNDMG LSLSVNYFKY LDYIFTCPIL TLDLLWSLNL P YKITYSLF ...String: MKTIIALSYI FCLVFADYKD DDDAMGTTSA PSLSDPNWQY GMGGWNNPRL PNFNLHDPTV IGVDWLGFLC LLGASLALMY KLMSFKGPD GDQEFFVGYR EEKCLSIYVN LIAAITYWGR ICAHFNNDMG LSLSVNYFKY LDYIFTCPIL TLDLLWSLNL P YKITYSLF VGLTIACGVF CNAFEPPARY LWFMFGCFIF AFTWISIIRL VYARFQQFLN EDAKKIRAPL KLSLTLYFSI WC GYPALWL LTEFGAISQL AAHVTTVIMD VAAKSVYGFA LLKFQLGVDK RDVWLDELKS VRYRDVVPQI RPSKTREGRM EYS EDGDFM RPSKGKRAEG DYMNPRWDHH DDGRRLPDSR EMDEQVHEKD QEISSTMKQI ADLNKQLSAM QESEAVENLY FQ UniProtKB: Cation channel rhodopsin 4 |
-Macromolecule #2: RETINAL
| Macromolecule | Name: RETINAL / type: ligand / ID: 2 / Number of copies: 1 / Formula: RET |
|---|---|
| Molecular weight | Theoretical: 284.436 Da |
| Chemical component information |  ChemComp-RET: |
-Macromolecule #3: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE
| Macromolecule | Name: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE / type: ligand / ID: 3 / Number of copies: 3 / Formula: PC1 |
|---|---|
| Molecular weight | Theoretical: 790.145 Da |
| Chemical component information |  ChemComp-PC1: |
-Macromolecule #4: water
| Macromolecule | Name: water / type: ligand / ID: 4 / Number of copies: 9 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 120 sec. | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV | |||||||||||||||
| Details | The protein was reconstituted in lipid nanodiscs. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 16366 / Average exposure time: 2.0 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.6 µm / Nominal defocus min: 0.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | Chain - Source name: AlphaFold / Chain - Initial model type: in silico model |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-8yel: |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)