+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of ET-1 bound ETBR-DNGI complex | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | ENDOTHELIN / RECEPTOR / Gi / COMPLEX / MEMBRANE PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationenteric smooth muscle cell differentiation / response to endothelin / positive regulation of prostaglandin-endoperoxide synthase activity / aldosterone metabolic process / negative regulation of neuron maturation / endothelin A receptor binding / protein kinase C deactivation / chordate pharynx development / phospholipase D-activating G protein-coupled receptor signaling pathway / rhythmic excitation ...enteric smooth muscle cell differentiation / response to endothelin / positive regulation of prostaglandin-endoperoxide synthase activity / aldosterone metabolic process / negative regulation of neuron maturation / endothelin A receptor binding / protein kinase C deactivation / chordate pharynx development / phospholipase D-activating G protein-coupled receptor signaling pathway / rhythmic excitation / endothelin receptor activity / peptide hormone secretion / endothelin B receptor binding / cellular response to human chorionic gonadotropin stimulus / meiotic cell cycle process involved in oocyte maturation / positive regulation of artery morphogenesis / semaphorin-plexin signaling pathway involved in axon guidance / body fluid secretion / neural crest cell fate commitment / regulation of fever generation / vein smooth muscle contraction / glomerular endothelium development / response to prostaglandin F / sympathetic neuron axon guidance / positive regulation of sarcomere organization / noradrenergic neuron differentiation / positive regulation of renal sodium excretion / histamine secretion / leukocyte activation / maternal process involved in parturition / positive regulation of chemokine-mediated signaling pathway / positive regulation of penile erection / rough endoplasmic reticulum lumen / neuroblast migration / heparin metabolic process / posterior midgut development / developmental pigmentation / pharyngeal arch artery morphogenesis / regulation of D-glucose transmembrane transport / positive regulation of odontogenesis / epithelial fluid transport / endothelin receptor signaling pathway involved in heart process / negative regulation of hormone secretion / cardiac neural crest cell migration involved in outflow tract morphogenesis / Weibel-Palade body / response to leptin / endothelin receptor signaling pathway / response to ozone / podocyte differentiation / positive regulation of cell growth involved in cardiac muscle cell development / renal sodium ion absorption / artery smooth muscle contraction / response to sodium phosphate / glomerular filtration / renal sodium excretion / protein transmembrane transport / enteric nervous system development / axonogenesis involved in innervation / positive regulation of cation channel activity / renin secretion into blood stream / cellular response to follicle-stimulating hormone stimulus / cellular response to luteinizing hormone stimulus / melanocyte differentiation / : / regulation of pH / positive regulation of prostaglandin secretion / renal albumin absorption / regulation of epithelial cell proliferation / respiratory gaseous exchange by respiratory system / basal part of cell / cellular response to mineralocorticoid stimulus / peripheral nervous system development / positive regulation of smooth muscle contraction / response to salt / positive regulation of urine volume / positive regulation of hormone secretion / negative regulation of adenylate cyclase activity / regulation of systemic arterial blood pressure by endothelin / vasoconstriction / negative regulation of blood coagulation / type 1 angiotensin receptor binding / embryonic heart tube development / : / dorsal/ventral pattern formation / axon extension / establishment of endothelial barrier / superoxide anion generation / positive regulation of neutrophil chemotaxis / positive regulation of signaling receptor activity / cartilage development / middle ear morphogenesis / cellular response to glucocorticoid stimulus / negative regulation of protein metabolic process / neural crest cell migration / cGMP-mediated signaling / prostaglandin biosynthetic process / cellular response to fatty acid / nitric oxide transport / positive regulation of heart rate / branching involved in blood vessel morphogenesis Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | ||||||||||||
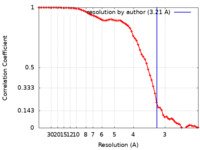
| Method | single particle reconstruction / cryo EM / Resolution: 3.21 Å | ||||||||||||
 Authors Authors | Tani K / Maki-Yonekura S / Kanno R / Negami T / Hamaguchi T / Hall M / Mizoguchi A / Humbel BM / Terada T / Yonekura K / Doi T | ||||||||||||
| Funding support |  Japan, 3 items Japan, 3 items
| ||||||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2024 Journal: Commun Biol / Year: 2024Title: Structure of endothelin ET receptor-G complex in a conformation stabilized by unique NPxxL motif. Authors: Kazutoshi Tani / Saori Maki-Yonekura / Ryo Kanno / Tatsuki Negami / Tasuku Hamaguchi / Malgorzata Hall / Akira Mizoguchi / Bruno M Humbel / Tohru Terada / Koji Yonekura / Tomoko Doi /  Abstract: Endothelin type B receptor (ETR) plays a crucial role in regulating blood pressure and humoral homeostasis, making it an important therapeutic target for related diseases. ETR activation by the ...Endothelin type B receptor (ETR) plays a crucial role in regulating blood pressure and humoral homeostasis, making it an important therapeutic target for related diseases. ETR activation by the endogenous peptide hormones endothelin (ET)-1-3 stimulates several signaling pathways, including G, G, G, G, and β-arrestin. Although the conserved NPxxY motif in transmembrane helix 7 (TM7) is important during GPCR activation, ETR possesses the lesser known NPxxL motif. In this study, we present the cryo-EM structure of the ETR-G complex, complemented by MD simulations and functional studies. These investigations reveal an unusual movement of TM7 to the intracellular side during ETR activation and the essential roles of the diverse NPxxL motif in stabilizing the active conformation of ETR and organizing the assembly of the binding pocket for the α5 helix of G protein. These findings enhance our understanding of the interactions between GPCRs and G proteins, thereby advancing the development of therapeutic strategies. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_38740.map.gz emd_38740.map.gz | 25.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-38740-v30.xml emd-38740-v30.xml emd-38740.xml emd-38740.xml | 20.6 KB 20.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_38740_fsc.xml emd_38740_fsc.xml | 6.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_38740.png emd_38740.png | 56.6 KB | ||
| Filedesc metadata |  emd-38740.cif.gz emd-38740.cif.gz | 6.5 KB | ||
| Others |  emd_38740_half_map_1.map.gz emd_38740_half_map_1.map.gz emd_38740_half_map_2.map.gz emd_38740_half_map_2.map.gz | 20.6 MB 20.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-38740 http://ftp.pdbj.org/pub/emdb/structures/EMD-38740 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38740 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38740 | HTTPS FTP |
-Validation report
| Summary document |  emd_38740_validation.pdf.gz emd_38740_validation.pdf.gz | 782.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_38740_full_validation.pdf.gz emd_38740_full_validation.pdf.gz | 781.9 KB | Display | |
| Data in XML |  emd_38740_validation.xml.gz emd_38740_validation.xml.gz | 12.3 KB | Display | |
| Data in CIF |  emd_38740_validation.cif.gz emd_38740_validation.cif.gz | 17.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38740 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38740 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38740 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38740 | HTTPS FTP |
-Related structure data
| Related structure data |  8xwpMC  8xwqC  8zrtC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_38740.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_38740.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.19 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_38740_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_38740_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : ET-1 BOUND ETBR-GI COMPLEX
| Entire | Name: ET-1 BOUND ETBR-GI COMPLEX |
|---|---|
| Components |
|
-Supramolecule #1: ET-1 BOUND ETBR-GI COMPLEX
| Supramolecule | Name: ET-1 BOUND ETBR-GI COMPLEX / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Endothelin receptor type B
| Macromolecule | Name: Endothelin receptor type B / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 38.808262 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: PGGGLAPAEV PKGDRTAGSP PRTISPPPCQ GPIEIKETFK YINTVVSCLV FVLGIIGNST LLYIIYKNKC MRNGPNILIA SLALGDLLH IVIDIPINVY KLLAEDWPFG AEMCKLVPFI QKASVGITVL SLCALSIDRY RAVASWSRIK GIGVPKWTAV E IVLIWVVS ...String: PGGGLAPAEV PKGDRTAGSP PRTISPPPCQ GPIEIKETFK YINTVVSCLV FVLGIIGNST LLYIIYKNKC MRNGPNILIA SLALGDLLH IVIDIPINVY KLLAEDWPFG AEMCKLVPFI QKASVGITVL SLCALSIDRY RAVASWSRIK GIGVPKWTAV E IVLIWVVS VVLAVPEAIG FDIITMDYKG SYLRICLLHP VQKTAFMQFY KTAKDWWLFS FYFCLPLAIT AFFYTLMTCE ML RKKSGMQ IALNDHLKQR REVAKTVFCL VLVFALCWLP LHLSRILKLT LYNQNDPNRC ELLSFLLVLD YIGINMASLN SCI NPIALY LVSKRFKNAF KSALCCWAQS UniProtKB: Endothelin receptor type B |
-Macromolecule #2: Endothelin-1
| Macromolecule | Name: Endothelin-1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 2.497951 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: CSCSSLMDKE CVYFCHLDII W UniProtKB: Endothelin-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(i) subunit alpha-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(i) subunit alpha-1 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.414047 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKNTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI ...String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKNTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI PTQQDVLRTR VKTTGIVETH FTFKDLHFKM FDVGAQRSER KKWIHCFEGV TAIIFCVALS DYDLVLAEDE EM NRMHASM KLFDSICNNK WFTDTSIILF LNKKDLFEEK IKKSPLTICY PEYAGSNTYE EAAAYIQCQF EDLNKRKDTK EIY THFTCS TDTKNVQFVF DAVTDVIIKN NLKDCGLF UniProtKB: Guanine nucleotide-binding protein G(i) subunit alpha-1 |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.671102 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: PGSSGSELDQ LRQEAEQLKN QIRDARKACA DATLSQITNN IDPVGRIQMR TRRTLRGHLA KIYAMHWGTD SRLLVSASQD GKLIIWDSY TTNKVHAIPL RSSWVMTCAY APSGNYVACG GLDNICSIYN LKTREGNVRV SRELAGHTGY LSCCRFLDDN Q IVTSSGDT ...String: PGSSGSELDQ LRQEAEQLKN QIRDARKACA DATLSQITNN IDPVGRIQMR TRRTLRGHLA KIYAMHWGTD SRLLVSASQD GKLIIWDSY TTNKVHAIPL RSSWVMTCAY APSGNYVACG GLDNICSIYN LKTREGNVRV SRELAGHTGY LSCCRFLDDN Q IVTSSGDT TCALWDIETG QQTTTFTGHT GDVMSLSLAP DTRLFVSGAC DASAKLWDVR EGMCRQTFTG HESDINAICF FP NGNAFAT GSDDATCRLF DLRADQELMT YSHDNIICGI TSVSFSKSGR LLLAGYDDFN CNVWDALKAD RAGVLAGHDN RVS CLGVTD DGMAVATGSW DSFLKIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #5: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #6: SCFV16
| Macromolecule | Name: SCFV16 / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 29.39893 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MVSAIVLYVL LAAAAHSAFA DVQLVESGGG LVQPGGSRKL SCSASGFAFS SFGMHWVRQA PEKGLEWVAY ISSGSGTIYY ADTVKGRFT ISRDDPKNTL FLQMTSLRSE DTAMYYCVRS IYYYGSSPFD FWGQGTTLTV SSGGGGSGGG GSGGGGSDIV M TQATSSVP ...String: MVSAIVLYVL LAAAAHSAFA DVQLVESGGG LVQPGGSRKL SCSASGFAFS SFGMHWVRQA PEKGLEWVAY ISSGSGTIYY ADTVKGRFT ISRDDPKNTL FLQMTSLRSE DTAMYYCVRS IYYYGSSPFD FWGQGTTLTV SSGGGGSGGG GSGGGGSDIV M TQATSSVP VTPGESVSIS CRSSKSLLHS NGNTYLYWFL QRPGQSPQLL IYRMSNLASG VPDRFSGSGS GTAFTLTISR LE AEDVGVY YCMQHLEYPL TFGAGTKLEL KGSLEVLFQG |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
| Details | This sample was monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 300 |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 3.4 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.5 µm / Nominal defocus min: 0.5 µm |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-8xwp: |
 Movie
Movie Controller
Controller




































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)