[English] 日本語
 Yorodumi
Yorodumi- EMDB-38075: CryoEM structure of the histamine H1 receptor-BRIL/Anti BRIL Fab ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of the histamine H1 receptor-BRIL/Anti BRIL Fab complex with astemizole | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationHistamine receptors / histamine receptor activity / cellular response to histamine / regulation of vascular permeability / G protein-coupled serotonin receptor activity / neurotransmitter receptor activity / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / positive regulation of vasoconstriction / electron transport chain / visual learning ...Histamine receptors / histamine receptor activity / cellular response to histamine / regulation of vascular permeability / G protein-coupled serotonin receptor activity / neurotransmitter receptor activity / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / positive regulation of vasoconstriction / electron transport chain / visual learning / regulation of synaptic plasticity / memory / phospholipase C-activating G protein-coupled receptor signaling pathway / chemical synaptic transmission / G alpha (q) signalling events / periplasmic space / electron transfer activity / inflammatory response / iron ion binding / G protein-coupled receptor signaling pathway / dendrite / heme binding / synapse / plasma membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Wang DD / Guo Q / Tao YY | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Molecular mechanism of antihistamines recognition and regulation of the histamine H receptor. Authors: Dandan Wang / Qiong Guo / Zhangsong Wu / Ming Li / Binbin He / Yang Du / Kaiming Zhang / Yuyong Tao /  Abstract: Histamine receptors are a group of G protein-coupled receptors (GPCRs) that play important roles in various physiological and pathophysiological conditions. Antihistamines that target the histamine H ...Histamine receptors are a group of G protein-coupled receptors (GPCRs) that play important roles in various physiological and pathophysiological conditions. Antihistamines that target the histamine H receptor (HR) have been widely used to relieve the symptoms of allergy and inflammation. Here, to uncover the details of the regulation of HR by the known second-generation antihistamines, thereby providing clues for the rational design of newer antihistamines, we determine the cryo-EM structure of HR in the apo form and bound to different antihistamines. In addition to the deep hydrophobic cavity, we identify a secondary ligand-binding site in HR, which potentially may support the introduction of new derivative groups to generate newer antihistamines. Furthermore, these structures show that antihistamines exert inverse regulation by utilizing a shared phenyl group that inserts into the deep cavity and block the movement of the toggle switch residue W428. Together, these results enrich our understanding of GPCR modulation and facilitate the structure-based design of novel antihistamines. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_38075.map.gz emd_38075.map.gz | 136.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-38075-v30.xml emd-38075-v30.xml emd-38075.xml emd-38075.xml | 13.8 KB 13.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_38075.png emd_38075.png | 82.3 KB | ||
| Filedesc metadata |  emd-38075.cif.gz emd-38075.cif.gz | 5.5 KB | ||
| Others |  emd_38075_half_map_1.map.gz emd_38075_half_map_1.map.gz emd_38075_half_map_2.map.gz emd_38075_half_map_2.map.gz | 134.4 MB 134.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-38075 http://ftp.pdbj.org/pub/emdb/structures/EMD-38075 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38075 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38075 | HTTPS FTP |
-Validation report
| Summary document |  emd_38075_validation.pdf.gz emd_38075_validation.pdf.gz | 706.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_38075_full_validation.pdf.gz emd_38075_full_validation.pdf.gz | 706.3 KB | Display | |
| Data in XML |  emd_38075_validation.xml.gz emd_38075_validation.xml.gz | 14.2 KB | Display | |
| Data in CIF |  emd_38075_validation.cif.gz emd_38075_validation.cif.gz | 16.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38075 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38075 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38075 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38075 | HTTPS FTP |
-Related structure data
| Related structure data |  8x5yMC  8x5xC  8x63C  8x64C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_38075.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_38075.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
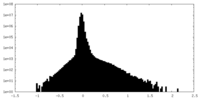
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_38075_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
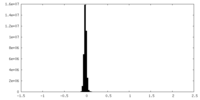
| Density Histograms |
-Half map: #1
| File | emd_38075_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : CryoEM structure of the histamine H1 receptor-BRIL/Anti BRIL Fab ...
| Entire | Name: CryoEM structure of the histamine H1 receptor-BRIL/Anti BRIL Fab complex with astemizole |
|---|---|
| Components |
|
-Supramolecule #1: CryoEM structure of the histamine H1 receptor-BRIL/Anti BRIL Fab ...
| Supramolecule | Name: CryoEM structure of the histamine H1 receptor-BRIL/Anti BRIL Fab complex with astemizole type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Histamine H1 receptor,Soluble cytochrome b562
| Macromolecule | Name: Histamine H1 receptor,Soluble cytochrome b562 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 50.629363 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DYKDDDDKTT MASPQLMPLV VVLSTICLVT VGLNLLVLYA VRSERKLHTV GNLYIVSLSV ADLIVGAVVM PMNILYLLMS KWSLGRPLC LFWLSMDYVA STASIFSVFI LCIDRYRSVQ QPLRYLKYRT KTRASATILG AWFLSFLWVI PILGWNHFMQ Q TSVRREDK ...String: DYKDDDDKTT MASPQLMPLV VVLSTICLVT VGLNLLVLYA VRSERKLHTV GNLYIVSLSV ADLIVGAVVM PMNILYLLMS KWSLGRPLC LFWLSMDYVA STASIFSVFI LCIDRYRSVQ QPLRYLKYRT KTRASATILG AWFLSFLWVI PILGWNHFMQ Q TSVRREDK CETDFYDVTW FKVMTAIINF YLPTLLMLWF YAKIYKAVKR QLADLEDNWE TLNDNLKVIE KADNAAQVKD AL TKMRAAA LDAQKASGSG SPEMKDFRHG FDILVGQIDD ALKLANEGKV KEAQAAAEQL KTTRNAYIQK YLKFCNRERK AAK QLGFIM AAFILCWIPY FIFFMVIAFC KNCCNEHLHM FTIWLGYINS TLNPLIYPLC NENFKKTFKR ILKIAALKEK IAAL KEKIA ALKEAEEKRA SRLEEELRRR LTEGSHHHHH HHH UniProtKB: Histamine H1 receptor, Soluble cytochrome b562 |
-Macromolecule #2: 1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxyphenyl)ethyl]piperid...
| Macromolecule | Name: 1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxyphenyl)ethyl]piperidin-4-yl}-1H-benzimidazol-2-amine type: ligand / ID: 2 / Number of copies: 1 / Formula: XB7 |
|---|---|
| Molecular weight | Theoretical: 458.57 Da |
| Chemical component information |  ChemComp-XB7: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: NITROGEN |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 357476 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)