[English] 日本語
 Yorodumi
Yorodumi- EMDB-36938: Outward-facing structure of human ABCB6 W546A mutant (ADP/VO4-bound) -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Outward-facing structure of human ABCB6 W546A mutant (ADP/VO4-bound) | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | ATP-Binding Cassette transporter / mitochondrial transporter / outward-facing state / ADP/VO4-bound / ABCB6 / MEMBRANE PROTEIN / TRANSPORT PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationcellular detoxification of cadmium ion / Defective ABCB6 causes MCOPCB7 / Mitochondrial ABC transporters / tetrapyrrole metabolic process / heme transmembrane transport / ABC-type heme transporter / ABC-type heme transporter activity / porphyrin-containing compound metabolic process / heme transport / tetrapyrrole binding ...cellular detoxification of cadmium ion / Defective ABCB6 causes MCOPCB7 / Mitochondrial ABC transporters / tetrapyrrole metabolic process / heme transmembrane transport / ABC-type heme transporter / ABC-type heme transporter activity / porphyrin-containing compound metabolic process / heme transport / tetrapyrrole binding / heme metabolic process / porphyrin-containing compound biosynthetic process / melanosome assembly / melanosome membrane / multivesicular body membrane / mitochondrial envelope / endolysosome membrane / vacuolar membrane / skin development / efflux transmembrane transporter activity / intracellular copper ion homeostasis / ABC-type transporter activity / ATP-binding cassette (ABC) transporter complex / brain development / transmembrane transport / early endosome membrane / intracellular iron ion homeostasis / mitochondrial outer membrane / endosome / lysosomal membrane / Golgi membrane / heme binding / endoplasmic reticulum membrane / Golgi apparatus / endoplasmic reticulum / ATP hydrolysis activity / mitochondrion / extracellular exosome / nucleoplasm / ATP binding / plasma membrane / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
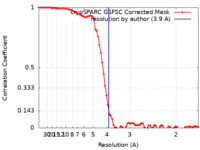
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | ||||||||||||
 Authors Authors | Jin MS / Lee SS / Park JG / Jang E / Choi SH / Kim S / Kim JW | ||||||||||||
| Funding support |  Korea, Republic Of, 3 items Korea, Republic Of, 3 items
| ||||||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2023 Journal: Commun Biol / Year: 2023Title: W546 stacking disruption traps the human porphyrin transporter ABCB6 in an outward-facing transient state. Authors: Sang Soo Lee / Jun Gyou Park / Eunhong Jang / Seung Hun Choi / Subin Kim / Ji Won Kim / Mi Sun Jin /  Abstract: Human ATP-binding cassette transporter subfamily B6 (ABCB6) is a mitochondrial ATP-driven pump that translocates porphyrins from the cytoplasm into mitochondria for heme biosynthesis. Within the ...Human ATP-binding cassette transporter subfamily B6 (ABCB6) is a mitochondrial ATP-driven pump that translocates porphyrins from the cytoplasm into mitochondria for heme biosynthesis. Within the transport pathway, a conserved aromatic residue W546 located in each monomer plays a pivotal role in stabilizing the occluded conformation via π-stacking interactions. Herein, we employed cryo-electron microscopy to investigate the structural consequences of a single W546A mutation in ABCB6, both in detergent micelles and nanodiscs. The results demonstrate that the W546A mutation alters the conformational dynamics of detergent-purified ABCB6, leading to entrapment of the transporter in an outward-facing transient state. However, in the nanodisc system, we observed a direct interaction between the transporter and a phospholipid molecule that compensates for the absence of the W546 residue, thereby facilitating the normal conformational transition of the transporter toward the occluded state following ATP hydrolysis. The findings also reveal that adoption of the outward-facing conformation causes charge repulsion between ABCB6 and the bound substrate, and rearrangement of key interacting residues at the substrate-binding site. Consequently, the affinity for the substrate is significantly reduced, facilitating its release from the transporter. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36938.map.gz emd_36938.map.gz | 43.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36938-v30.xml emd-36938-v30.xml emd-36938.xml emd-36938.xml | 15.4 KB 15.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_36938_fsc.xml emd_36938_fsc.xml | 7.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_36938.png emd_36938.png | 20.5 KB | ||
| Filedesc metadata |  emd-36938.cif.gz emd-36938.cif.gz | 5.7 KB | ||
| Others |  emd_36938_half_map_1.map.gz emd_36938_half_map_1.map.gz emd_36938_half_map_2.map.gz emd_36938_half_map_2.map.gz | 43.1 MB 43.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36938 http://ftp.pdbj.org/pub/emdb/structures/EMD-36938 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36938 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36938 | HTTPS FTP |
-Validation report
| Summary document |  emd_36938_validation.pdf.gz emd_36938_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_36938_full_validation.pdf.gz emd_36938_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_36938_validation.xml.gz emd_36938_validation.xml.gz | 15.6 KB | Display | |
| Data in CIF |  emd_36938_validation.cif.gz emd_36938_validation.cif.gz | 19.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36938 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36938 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36938 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36938 | HTTPS FTP |
-Related structure data
| Related structure data |  8k7cMC  8k7bC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36938.map.gz / Format: CCP4 / Size: 46.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36938.map.gz / Format: CCP4 / Size: 46.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.851 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_36938_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
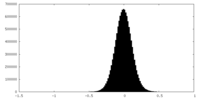
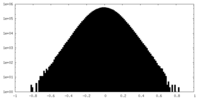
| Density Histograms |
-Half map: #1
| File | emd_36938_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
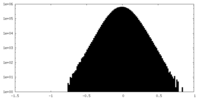
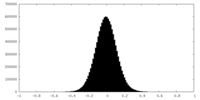
| Density Histograms |
- Sample components
Sample components
-Entire : ATP-binding cassette sub-family B member 6 - Homo sapiens
| Entire | Name: ATP-binding cassette sub-family B member 6 - Homo sapiens |
|---|---|
| Components |
|
-Supramolecule #1: ATP-binding cassette sub-family B member 6 - Homo sapiens
| Supramolecule | Name: ATP-binding cassette sub-family B member 6 - Homo sapiens type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: ATP-binding cassette sub-family B member 6
| Macromolecule | Name: ATP-binding cassette sub-family B member 6 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: ABC-type heme transporter |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 65.869797 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: RDFGRKLRLL SGYLWPRGSP ALQLVVLICL GLMGLERALN VLVPIFYRNI VNLLTEKAPW NSLAWTVTSY VFLKFLQGGG TGSTGFVSN LRTFLWIRVQ QFTSRRVELL IFSHLHELSL RWHLGRRTGE VLRIADRGTS SVTGLLSYLV FNVIPTLADI I IGIIYFSM ...String: RDFGRKLRLL SGYLWPRGSP ALQLVVLICL GLMGLERALN VLVPIFYRNI VNLLTEKAPW NSLAWTVTSY VFLKFLQGGG TGSTGFVSN LRTFLWIRVQ QFTSRRVELL IFSHLHELSL RWHLGRRTGE VLRIADRGTS SVTGLLSYLV FNVIPTLADI I IGIIYFSM FFNAWFGLIV FLCMSLYLTL TIVVTEWRTK FRRAMNTQEN ATRARAVDSL LNFETVKYYN AESYEVERYR EA IIKYQGL EWKSSASLVL LNQTQNLVIG LGLLAGSLLC AYFVTEQKLQ VGDYVLFGTY IIQLYMPLNA FGTYYRMIQT NFI DMENMF DLLKEETEVK DLPGAGPLRF QKGRIEFENV HFSYADGRET LQDVSFTVMP GQTLALVGPS GAGKSTILRL LFRF YDISS GCIRIDGQDI SQVTQASLRS HIGVVPQDTV LFNDTIADNI RYGRVTAGND EVEAAAQAAG IHDAIMAFPE GYRTQ VGER GLKLSGGEKQ RVAIARTILK APGIILLDEA TSALDTSNER AIQASLAKVC ANRTTIVVAH RLSTVVNADQ ILVIKD GCI VERGRHEALL SRGGVYADMW QLQQ UniProtKB: ATP-binding cassette sub-family B member 6 |
-Macromolecule #2: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 2 / Number of copies: 2 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #3: VANADATE ION
| Macromolecule | Name: VANADATE ION / type: ligand / ID: 3 / Number of copies: 2 / Formula: VO4 |
|---|---|
| Molecular weight | Theoretical: 114.939 Da |
| Chemical component information |  ChemComp-VN3: |
-Macromolecule #4: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 4 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4 mg/mL |
|---|---|
| Buffer | pH: 7 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.7000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z
Z Y
Y X
X