[English] 日本語
 Yorodumi
Yorodumi- EMDB-35679: Endogenous substrate adenine bound state of Arabidopsis AZG1 at pH 5.5 -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Endogenous substrate adenine bound state of Arabidopsis AZG1 at pH 5.5 | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | cytokinin / transporter / TRANSPORT PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationadenine transport / guanine transport / purine nucleobase transmembrane transporter activity / purine nucleobase transport / membrane / cytosol Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.6 Å | ||||||||||||
 Authors Authors | Xu L / Guo J | ||||||||||||
| Funding support |  China, 3 items China, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Plants / Year: 2024 Journal: Nat Plants / Year: 2024Title: Structures and mechanisms of the Arabidopsis cytokinin transporter AZG1. Authors: Lingyi Xu / Wei Jia / Xin Tao / Fan Ye / Yan Zhang / Zhong Jie Ding / Shao Jian Zheng / Shuai Qiao / Nannan Su / Yu Zhang / Shan Wu / Jiangtao Guo /  Abstract: Cytokinins are essential for plant growth and development, and their tissue distributions are regulated by transmembrane transport. Recent studies have revealed that members of the 'Aza-Guanine ...Cytokinins are essential for plant growth and development, and their tissue distributions are regulated by transmembrane transport. Recent studies have revealed that members of the 'Aza-Guanine Resistant' (AZG) protein family from Arabidopsis thaliana can mediate cytokinin uptake in roots. Here we present 2.7 to 3.3 Å cryo-electron microscopy structures of Arabidopsis AZG1 in the apo state and in complex with its substrates trans-zeatin (tZ), 6-benzyleaminopurine (6-BAP) or kinetin. AZG1 forms a homodimer and each subunit shares a similar topology and domain arrangement with the proteins of the nucleobase/ascorbate transporter (NAT) family. These structures, along with functional analyses, reveal the molecular basis for cytokinin recognition. Comparison of the AZG1 structures determined in inward-facing conformations and predicted by AlphaFold2 in the occluded conformation allowed us to propose that AZG1 may carry cytokinins across the membrane through an elevator mechanism. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35679.map.gz emd_35679.map.gz | 48.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35679-v30.xml emd-35679-v30.xml emd-35679.xml emd-35679.xml | 14.7 KB 14.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_35679.png emd_35679.png | 91.5 KB | ||
| Filedesc metadata |  emd-35679.cif.gz emd-35679.cif.gz | 5.8 KB | ||
| Others |  emd_35679_half_map_1.map.gz emd_35679_half_map_1.map.gz emd_35679_half_map_2.map.gz emd_35679_half_map_2.map.gz | 47.1 MB 47 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35679 http://ftp.pdbj.org/pub/emdb/structures/EMD-35679 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35679 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35679 | HTTPS FTP |
-Related structure data
| Related structure data |  8irmMC  8irlC  8irnC  8iroC  8irpC  8wmqC  8wo7C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_35679.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35679.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.851 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_35679_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
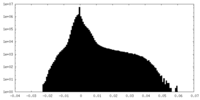
| Projections & Slices |
| ||||||||||||
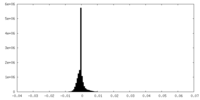
| Density Histograms |
-Half map: #2
| File | emd_35679_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
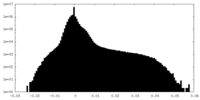
| Projections & Slices |
| ||||||||||||
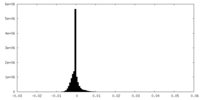
| Density Histograms |
- Sample components
Sample components
-Entire : complex of AZG1 and Adenine
| Entire | Name: complex of AZG1 and Adenine |
|---|---|
| Components |
|
-Supramolecule #1: complex of AZG1 and Adenine
| Supramolecule | Name: complex of AZG1 and Adenine / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 131.2 KDa |
-Macromolecule #1: Adenine/guanine permease AZG1
| Macromolecule | Name: Adenine/guanine permease AZG1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 65.686703 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MEQQQQQQLP STTTRPKPKL LNRLNTYVGS SRVGKRFKLA ERNSTFTTEL RAGTATFLTM AYILAVNASI LSDSGGTCSV SDCIPLCSN PAIEPSQCTG PGLRLIQPDV SCKFNPVNPG YAACVEEIRK DLIVATVAAS LIGCVIMGLM ANLPLALAPG M GTNAYFAY ...String: MEQQQQQQLP STTTRPKPKL LNRLNTYVGS SRVGKRFKLA ERNSTFTTEL RAGTATFLTM AYILAVNASI LSDSGGTCSV SDCIPLCSN PAIEPSQCTG PGLRLIQPDV SCKFNPVNPG YAACVEEIRK DLIVATVAAS LIGCVIMGLM ANLPLALAPG M GTNAYFAY TVVGFHGSGS ISYRTALAAV FIEGLIFLFI SAIGFRAKLA KLVPKPVRIS SSAGIGLFLA FIGLQNNQGI GL VGYSPST LVTLAACPAS SRISLAPVIT SANGTVSLLA GGSVSGDIMC IHGRMESPTF WLGIVGFVII AYCLVKNVKG AMI YGIVFV TAVSWFRNTE VTAFPNTSAG DAAHDYFKKI VDVHVIKHTA GALSFSGINK GHFWEALVTF LYVDILDTTG TLYS MARFA GFVDEKGDFA GQYFAFMSDA SAIVIGSLLG TSPVTVFIES STGIREGGRT GLTAITVAVY FLLAMFFTPL LASIP AWAV GPPLILVGVM MMKSVTEIDW EDMREAIPAF VTMILMPLTY SVAYGLIGGI GSYVVLHLWD WGEEGLVKLG FLKRKV KEE DNNNGVVKAS EIDTTVSGRD YKDDDDKWSH PQFEKGGGGS GGSAWSHPQF EK UniProtKB: Adenine/guanine permease AZG1 |
-Macromolecule #3: ADENINE
| Macromolecule | Name: ADENINE / type: ligand / ID: 3 / Number of copies: 2 / Formula: ADE |
|---|---|
| Molecular weight | Theoretical: 135.127 Da |
| Chemical component information |  ChemComp-ADE: |
-Macromolecule #4: water
| Macromolecule | Name: water / type: ligand / ID: 4 / Number of copies: 2 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 5.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 52.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.6 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.6 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 235460 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller









 Z
Z Y
Y X
X